Frostburg State University
General Chemistry Online: How Can Molarity Be Converted
Resource provides information about converting molarity to normality.
Science Education Resource Center at Carleton College
Serc:calculate the Molarity of Lemonade Solutions
In this lab activity, students taste different samples of lemonade - rate them and then determine the molarity of sugar and citric acid in the lemonade.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molarity and Equation Stoichiometry: Audio Book
This audio book, narrated by author Mark Bishop, describes how to use equation stoichiometry for reactions that are in a solution. Many examples are shown, and general steps to complete the problems are given.
Crescent Public Schools
The Internet Science Room: Solution Concentration
Students have the opportunity to study examples and worked out example problems to further their understanding of chemical solution concentration.
Khan Academy
Khan Academy: Molarity vs.osmolarity
Molarity and osmolarity are two distinct concepts. Molarity (M) is the number of moles of solute per liter of solution. The unit of molarity is the mole (mol). Osmolarity (Osm/L) is the total concentration of all solutes in the solution....
Khan Academy
Khan Academy: Molarity vs. Molality
Learn how molarity and molality differ. The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of a solution is equal to the moles of solute divided by the volume of...
Khan Academy
Khan Academy: Molarity, Molality, Osmolarity, Osmolality, and Tonicity What's the Difference?
See how each of these terms tells us something different about a solution.
Sophia Learning
Sophia: Molarity: Lesson 1
This lesson introduces molarity and explains what units are used to label solutions of various concentrations. It is 1 of 3 in the series titled "Molarity."
Other
Molarity Practice Worksheet [Pdf]
A worksheet with molarity practice problems accompanied by their solutions.
ClassFlow
Class Flow: Solubility and Solution
[Free Registration/Login Required] This flipchart explains the concepts of solubility, saturated, unsaturated, supersaturated, the factors affecting solubility, and molarity concentration calculations.
Chemistry Collective
Chem Collective: Cola and Sucrose Concentration Problem
In this activity, students use the virtual lab to prepare a sucrose solution for a soda recipe. They next calculate the concentration of their solution in terms of molarity, percent mass and density. Finally, they compare the density of...
Chemistry Collective
Chem Collective: Glucose Dilution Problem
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M...
Chemistry Collective
Chem Collective: Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to mix together to create...
Chemistry Collective
Chem Collective: Determination of the P H Scale
This virtual lab activity where students perform the method of successive dilutions using HCl, NaOH, a pH meter, and universal indicator solution to help understand the logarithmic nature of the pH scale.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Beer's Law Lab
An interactive simulation that teaches about Beer's Law, solutions, concentration, spectrophotometry, and more through observations to explore how much light is absorbed and transmitted with changes in thickness and color. This...
Chem Tutor
Chem Tutor: Concentration
An overall summary of the major units of the concentration of a solution, including normality.
ClassFlow
Class Flow: The Chemistry of Solutions
[Free Registration/Login Required] This flipchart covers the high school chemistry concept of solutions, types of solutions, solution concentration, molarity, percent concentration, Henry's law, solubility, and solubility curve.
Dartmouth College
Dartmouth College: Chem Lab: Calorimetry 1 Enthalpy of Formation of Mg O
In this lab experiment students are asked to determine the standard molar enthalpy of formation of magnesium oxide, MgO. Students are asked to use Hess's Law and the enthalpy changes of several other solution reactions during this...
University of Colorado
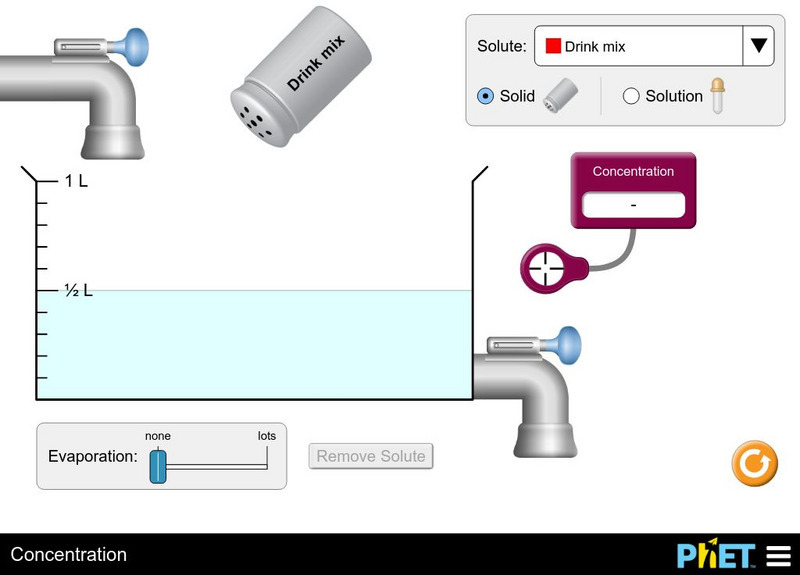
University of Colorado: Ph Et Interactive Simulations: Concentration
Watch your solution change color as you mix chemicals with water. Then check molarity with the concentration meter. What are all the ways you can change the concentration of your solution? Switch solutes to compare different chemicals...
ClassFlow
Class Flow: Acid Base Titration
[Free Registration/Login Required] Determination of the Molarity of an Acid or Base Solution.
Sophia Learning
Sophia: Molality: Lesson 2
This lesson introduces molality as a unit of concentration. Distinguish from molarity as a unit.
Cosmo Learning
Cosmo Learning: Junior Chemistry With Chemguy
A collection of video lectures to teach junior high students topics in chemistry. Lectures cover topics in understanding the periodic table, compounds, chemical bonding, Avogadro's number, balancing chemical equations, stoichiometry,...
Davidson College
Davidson College: Calorimetry:heat of Solution of Sulfuric Acid
An animation to investigate the rates of heating or cooling in a calorimeter. Also, molar enthalpy of solution of sulfuric acid is discovered.
Frostburg State University
General Chemistry Online: The Mole Concept
Resource provides notes on Moles and Stoichiometry. Deals with all book-keeping aspects, including as section on yields and limiting reactants. Includes lesson plans, lecture slides and notes, links to related websites, and frequently...