Hi, what do you want to do?
Curated OER
Acids and Bases: Together again!
Students identify principles behind acid-base reactions. They predict factors that may affect an acid-base reaction. Students identify questions and concepts that guide scientific investigations.
Curated OER
Types of Chemical Reactions
In this chemical reactions instructional activity, students are given directions about three types of reactions and how to write balanced equations for each. They then are given ten practice problems to complete using the information given.
Curated OER
Graham's Laws: Diffusion and Effusion of Gases
Young scholars conduct a series of experiments to explore Graham's law. In this chemistry instructional activity, students differentiate effusion and diffusion. They perform calculations using Boyle's, Henry's, Charles' and Graham's Laws.
Curated OER
Solutions and Solubility Review
In this solutions and solubility review worksheet, students are given main ideas about intermolecular forces, concentrations of solutions, molar solutions, ionic equations, solubility rules, acids and bases and titrations. Students...
Curated OER
Unit Conversion in Chemistry
Hopefully by the time high schoolers are enrolled in your chemistry class, they are skilled with unit conversion. It does not hurt, however, to take an hour early in the school year to review this vital skill. This plan simply provides a...
Curated OER
Necessary Nitrogen
Students view a video that presents the biogeochemical cycle of nitrogen. They compare types of soils and consider how different fertilizers affect soil composition.
Curated OER
Lactose & Lactase
Students examine the characteristics of lactose and lactase. In this enzyme lesson students study the lactose in milk and its rate of dissolution.
Curated OER
You say Avocado, I say Avogadro
Students convert mole to mass to particles and vice versa. In this chemistry lesson, students discuss the importance of Avogadro's number. They apply what they have learned in a team competition.
Clackamas Community College
Clackamas Community College: Molecular Formulas
Discussion of molecular formula and how to determine it from empirical formula. Practice problems are available.
University of Nebraska
University of Nebraska: Determining a Molecular Formula
This is a seven part site from the University of Nebraska on molecular formulas. Sections are: Introduction, Purpose, General Safety Considerations, Procedure, Data Analysis and Concept Development, Implications and Applications,...
CK-12 Foundation
Ck 12: Percent Composition and Empirical & Molecular Formulas
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will learn to calculate the percent composition of a compound either from mass data or from the chemical formula. They will use...
CK-12 Foundation
Ck 12: Chemistry: Molecular Formula
[Free Registration/Login may be required to access all resource tools.] Defines molecular formula and describes how to write molecular formulas.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Determination of Empirical and Molecular Formulas:
Listen as this audio book shows the determination of empirical and molecular formulas. View the formula to calculate empirical and molecular formulas and the written steps for calculating.
CK-12 Foundation
Ck 12: Chemical Formulas
[Free Registration/Login may be required to access all resource tools.] Students will use a chemical formula or mass data to calculate the percent composition of a compound, and then calculate the empirical or molecular formula for a...
Towson University
Towson University: Determining Formulae
Explanation of how to calculate empirical and molecular formulas.
Michael Blaber, PhD
Florida State Univ.: Stoichiometry: Chemical Formulas and Equations
Determining empirical formula from analytical data, and determing molecular formula from empirical formula, with flow charts and sample calculations.
Texas Education Agency
Texas Gateway: Empirical Formula
This tutorial reviews how to calculate molecular formula by giving students video, exercises, and practice.
CK-12 Foundation
Ck 12: Plix: Molecular Formula
[Free Registration/Login Required] In this interactive you will move the end of each arrow to connect the label with the correct model of each molecule. You will need a sign-in to access this media, but it will be well worth your time!
Other
University of South Carolina: Molecular Formula
A brief explanation of the relationship between molecular formula and empirical formula.
Other
Seton Hall University: Using Mass Percent
Slides in presentation show how to use mass percent to calculate empirical and molecular formulas.
Texas A&M University
Texas A&m: Steps for Determining an Empirical Formula
The steps for determining an empirical formula are listed on this site. Also, it tells how to determine the molecular formula from an empirical formula. Two example problems are worked through step by step.
CK-12 Foundation
Ck 12: Physical Science: Chemical Formula
[Free Registration/Login may be required to access all resource tools.] How to write chemical formulae and what they represent about the substance.
Texas Education Agency
Texas Gateway: Atomic and Molecular Explanation of Pressure and Temperature
We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Learn more about Kinetic Theory with the following detailed resource...
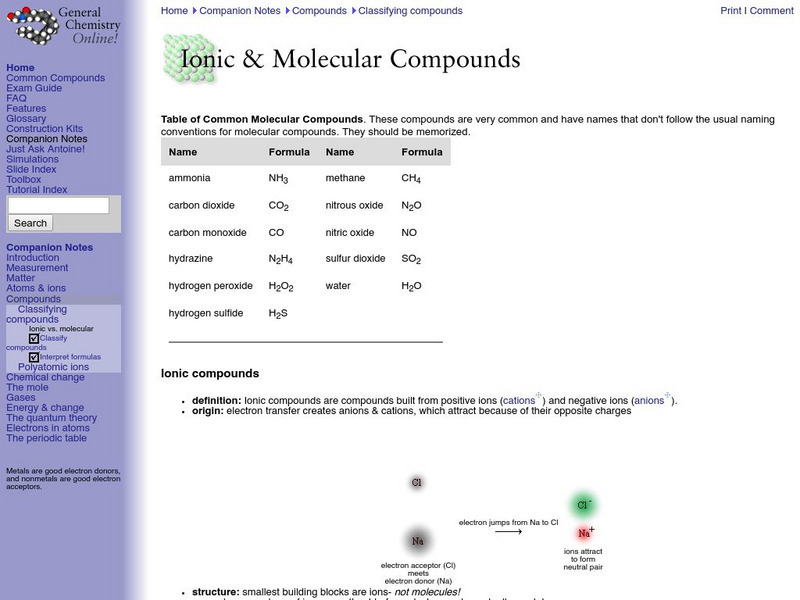
Frostburg State University
General Chemistry Online: Ionic and Molecular Compounds
Provides a good outline of the concepts involved in ionic and covalent bonding, with links to definition of terms. Features a list of common molecular compounds and a chart that compares ionic and molecular compounds.
Other popular searches
- Molecular Formula Mass
- Molecular Formula Combustion
- Molecular Formula Structural
- Writing Molecular Formulas
- Chemistry Molecular Formulas