Hi, what do you want to do?
CK-12 Foundation
Ck 12: Percent Composition and Empirical & Molecular Formulas
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will learn to calculate the percent composition of a compound either from mass data or from the chemical formula. They will use...
Clackamas Community College
Clackamas Community College: Molecular Formulas
Discussion of molecular formula and how to determine it from empirical formula. Practice problems are available.
CK-12 Foundation
Ck 12: Chemical Formulas
[Free Registration/Login may be required to access all resource tools.] Students will use a chemical formula or mass data to calculate the percent composition of a compound, and then calculate the empirical or molecular formula for a...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Determination of Empirical and Molecular Formulas:
Listen as this audio book shows the determination of empirical and molecular formulas. View the formula to calculate empirical and molecular formulas and the written steps for calculating.
Michael Blaber, PhD
Florida State Univ.: Stoichiometry: Chemical Formulas and Equations
Determining empirical formula from analytical data, and determing molecular formula from empirical formula, with flow charts and sample calculations.
Towson University
Towson University: Determining Formulae
Explanation of how to calculate empirical and molecular formulas.
Other
Seton Hall University: Using Mass Percent
Slides in presentation show how to use mass percent to calculate empirical and molecular formulas.
Crescent Public Schools
The Internet Science Room: Percentage Composition and Empirical Formulas
Students learn how to find percentage composition, the percentage of a formula mass represented by each element. Percentage composition compares the mass of one part of a substance to the mass of the whole.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molar Mass and Chemical Compounds: Audio Book
Listen and watch as this audio book shows the relations of molar mass and chemical compounds. Discover the formula for molecular mass, ionic compounds, and formula units, and view several pictures about chemical formulas.
Chiral Publishing
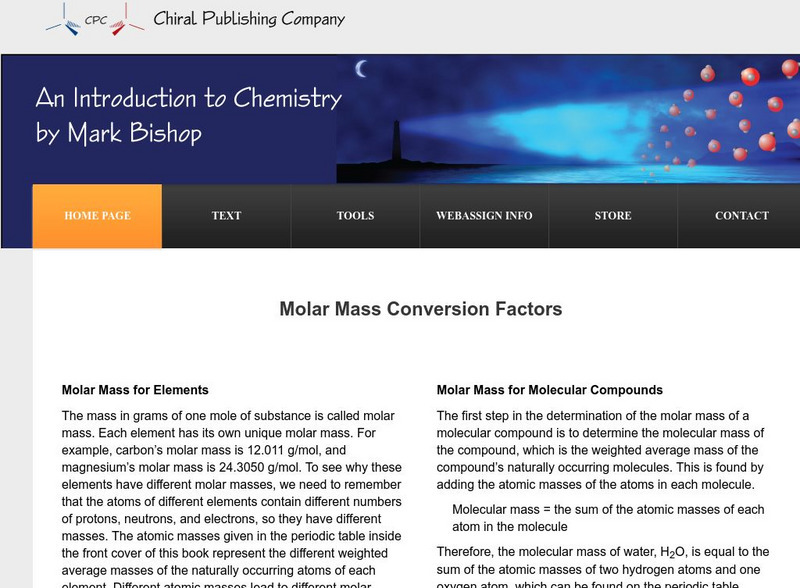
Chiral Publishing: An Introduction to Chemistry: Molar Mass Conversion Factors
Read how to use molar mass equations to find conversion factors and molecular compounds. View several examples using given formulas for molar mass.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Stoichiometry
This online textbook chapter provides learners with advanced-level reading and practice material on stoichiometry
Other
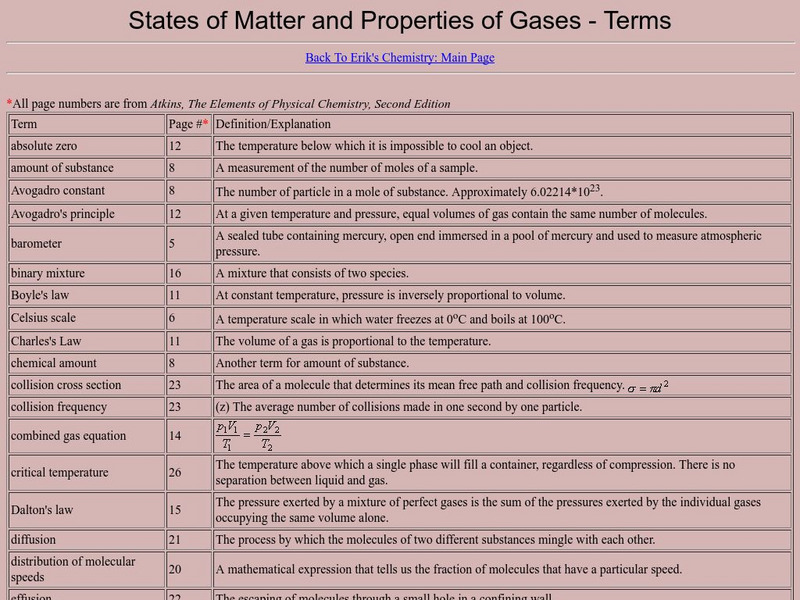
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.
Chemistry Collective
Chem Collective: Mixed Reception
Participate in the investigation of a virtual crime scene using chemistry concepts to solve a mysterious death. The 40-50 minute activity can be used as a classroom lab or as a homework assignment. Additionally, teachers may request a...
Frostburg State University
General Chemistry Online: Faq the Mole Concept
This FAQ site over general chemistry concepts presents the question: "Why are moles used?" It explains what moles are and how they can be used to count molecules and how grams can be turned into moles.