Khan Academy
Khan Academy: The Quantum Mechanical Model of the Atom
An explanation using quantum mechanics to describe the atom.
CK-12 Foundation
Ck 12: The Quantum Mechanical Model
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will calculate the wavelength, frequency, and energy of light using Planck's constant and the speed of light. They will...
Other
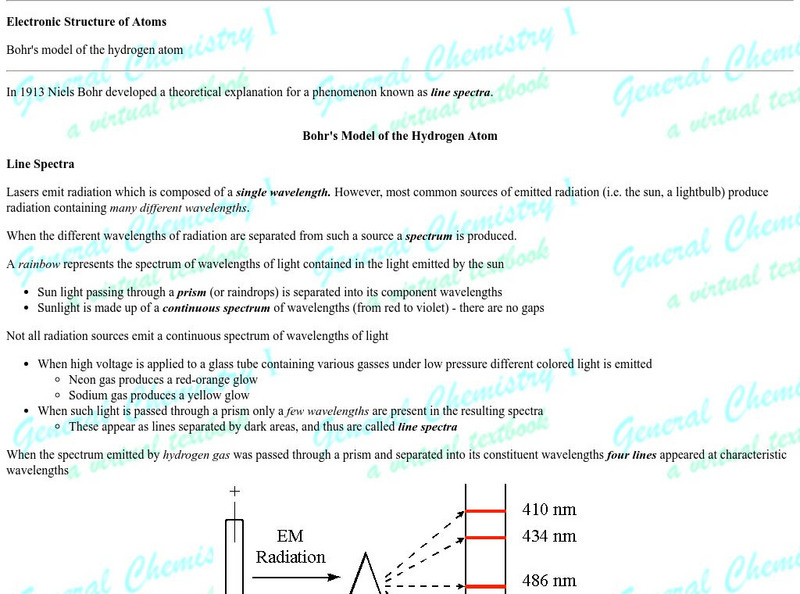
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Science Education Resource Center at Carleton College
Serc: Quantum Atomic Structure
This multi-day lesson plan helps young scholars to understand how the models of the atom have changed and how quantum mechanics affects the electrons as they orbit the nucleus.
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Michael Blaber, PhD
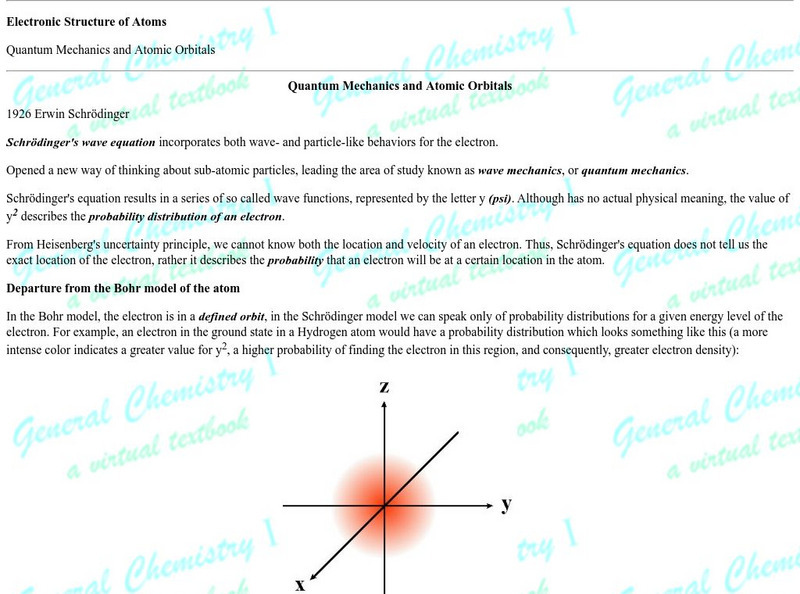
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
University of Maryland
Um: Quantum Mechanics of One and Two Electron Atoms
An explanation of Hund's rule and how to apply it to quantum mechanics. A mathematical-based explanation.
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Ohio State University
Betha Chemistry Tutorial
This site resource offers tutorials on the gas laws, balancing chemical equations, and quantum mechanics.
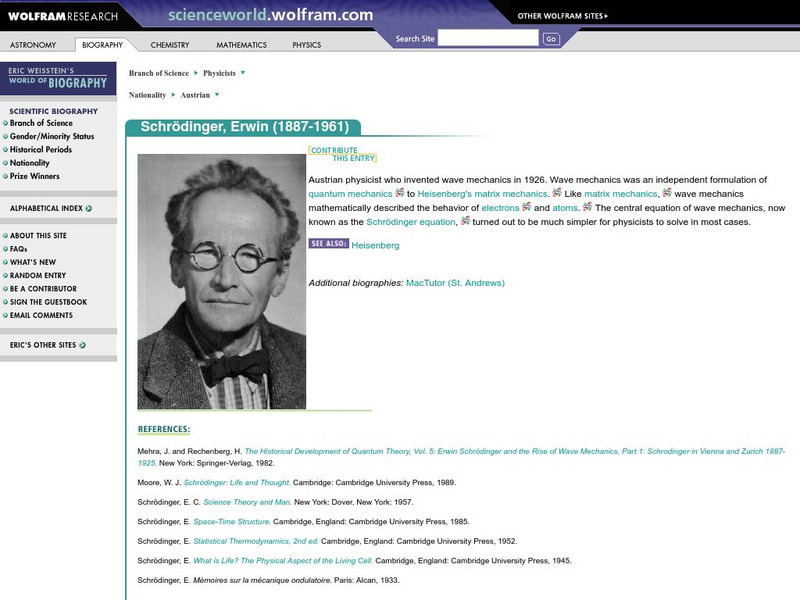
Wolfram Research
Wolfram Science World: Erwin Schrodinger
ScienceWorld.com offers a brief biography and image of Austrian physicist and inventor of wave mechanics, Erwin Schrodinger.
Lawrence Berkeley National Laboratory
Berkeley Lab: The Particle Adventure
Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Lasers
Learn about absorption and emission by creating a laser. Pump the chamber with a photon beam, and manipulate the energy states of the laser's atoms to control its output.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Lasers
Create a laser by pumping the chamber with a photon beam. Manage the energy states of the laser's atoms to control its output.
PBS
Pbs: A Science Odyssey
Website for the PBS series "A Science Odyssey." Numerous opportunities to explore the people and discoveries of science.
CK-12 Foundation
Ck 12: Physics Simulation: Atomic Colors
[Free Registration/Login Required] Explore the Bohr model of atoms by studying the absorption and emission of light from simple gases in the context of starlight using this interactive simulation. A PDF worksheet and a video tutorial are...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Rutherford Scattering
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining...
Wikimedia
Wikipedia: Absolute Zero
Wikipedia offers several paragraphs of detailed information on absolute zero, the lowest temperature that can be obtained in any macroscopic system.
CK-12 Foundation
Ck 12: Flex Book Textbooks: Chemistry Second Edition
[Free Registration/Login may be required to access all resource tools.] A complete, web-based, multi-media textbook covering a wide variety of Chemistry concepts.
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...