Hi, what do you want to do?
Curated OER
Historical Development of a Scientific Idea
Ninth graders examine how scientist's contribute to atomic theory. For this development of a scientific idea lesson students work in groups and research the development of the atomic model.
Curated OER
Energy Dispersive Spectroscopy
Students calculate the values of electron binding energies. In this physics lesson, students solve for different wavelength characteristics of X-rays. They present their findings to the class.
Khan Academy
Khan Academy: The Quantum Mechanical Model of the Atom
An explanation using quantum mechanics to describe the atom.
Khan Academy
Khan Academy: The Quantum Mechanical Model of the Atom
Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de Broglie wavelength, the Schrodinger equation, and the Heisenberg uncertainty principle. Electron spin and the...
TED Talks
Ted: Ted Ed: Particles and Waves: The Central Mystery of Quantum Mechanics
One of the most amazing facts in physics is that everything in the universe, from light to electrons to atoms, behaves like both a particle and a wave at the same time. But how did physicists arrive at this mind-boggling conclusion? Chad...
CK-12 Foundation
Ck 12: The Quantum Mechanical Model
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will calculate the wavelength, frequency, and energy of light using Planck's constant and the speed of light. They will...
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Quantum Wave Interference
When do photons, electrons, and atoms behave like particles and when do they behave like waves? In this interactive, use quantum detectors to explore how measurements change the waves and the patterns they produce on the screen.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Models of the Hydrogen Atom
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
Other
Quantum Mysteries: Deepening Quantum Mysteries
Although it is arguably the most successful theory in physics, quantum physics has often baffled and confounded both its practitioners and the general public. This informative website discusses the types of mysteries, which still plague...
Science Education Resource Center at Carleton College
Serc: Quantum Atomic Structure
This multi-day lesson plan helps students to understand how the models of the atom have changed and how quantum mechanics affects the electrons as they orbit the nucleus.
University of St. Andrews (UK)
University of St. Andrews: The Quantum Age Begins
This University of St. Andrews page provides an interesting discussion of the beginning of the age of quantum physics. (Quantum physics/mechanics is thought to govern the energy levels of electrons in atoms.) There is a references link...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Michael Blaber, PhD
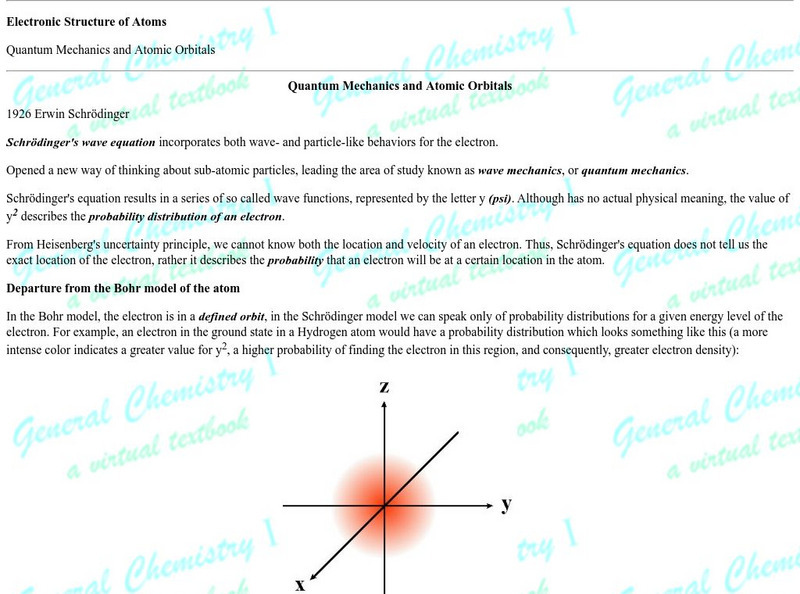
Fsu: Electronic Structure of Atoms: Quantum Mechanics & Atomic Orbitals
This article is provided by Florida State University. Schroedinger wave equation is explained and how quantum numbers are used to describe the energy level of any electron in an atom. The relationship of atomic orbitals to quantum...
University of Maryland
Um: Quantum Mechanics of One and Two Electron Atoms
An explanation of Hund's rule and how to apply it to quantum mechanics. A mathematical-based explanation.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Stern Gerlach Experiment
An interactive simulation that teaches about quantum mechanics, spin, and quantum measurement through a classic Stern-Gerlach Experiment. By observing the spin of atoms as an intrinsic angular momentum, students learn to measure to reach...
Other
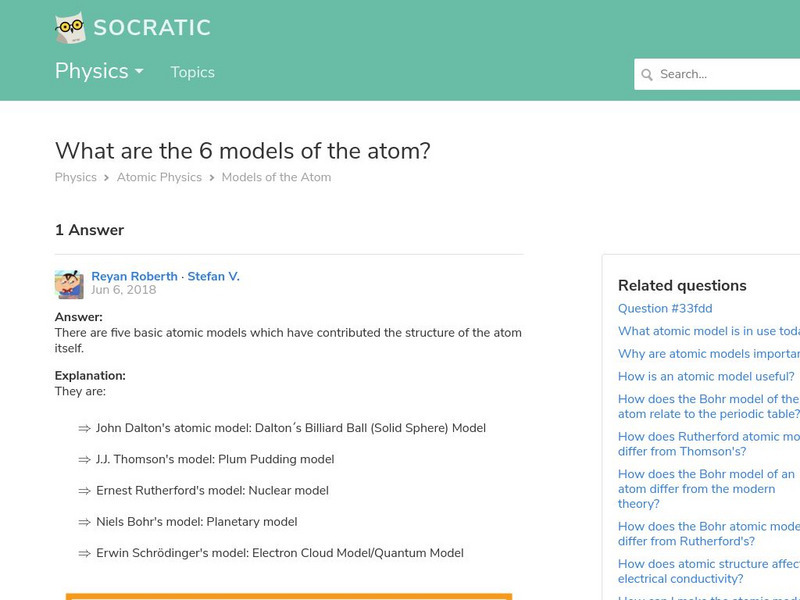
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
PBS
Pbs: Planck Discovers the Quantum Nature of Energy
PBS offers a short summary of the discovery of the quantum nature of the atom by Max Planck. Easy to follow.
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Ohio State University
Betha Chemistry Tutorial
This site resource offers tutorials on the gas laws, balancing chemical equations, and quantum mechanics.
University of Colorado
University of Colorado: Physics 2000: Schrodinger's Atom
A simple dialogue to explain quantum theory and the Schrodinger equation. Includes several java applets.
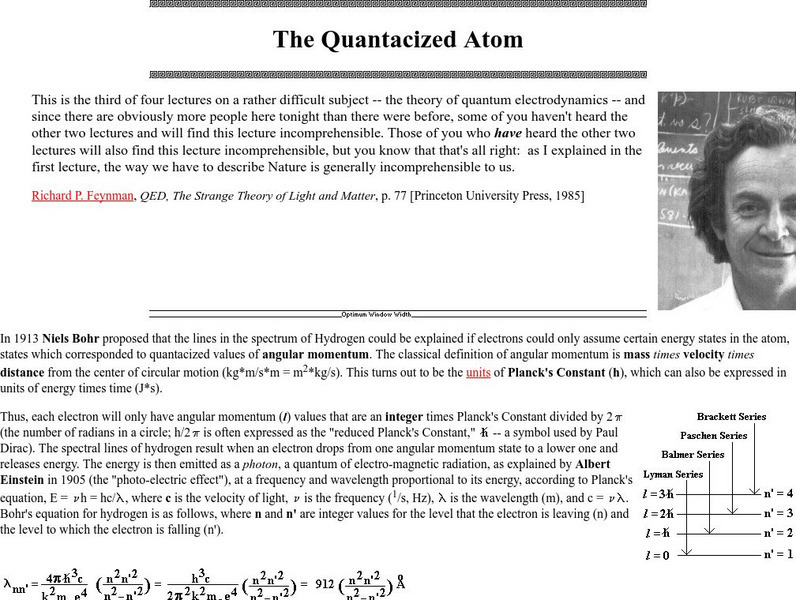
Friesian School
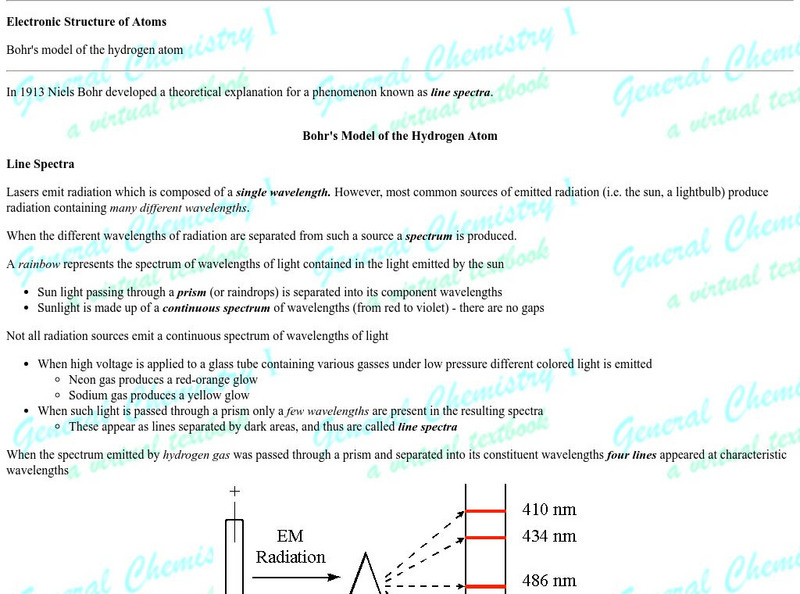
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
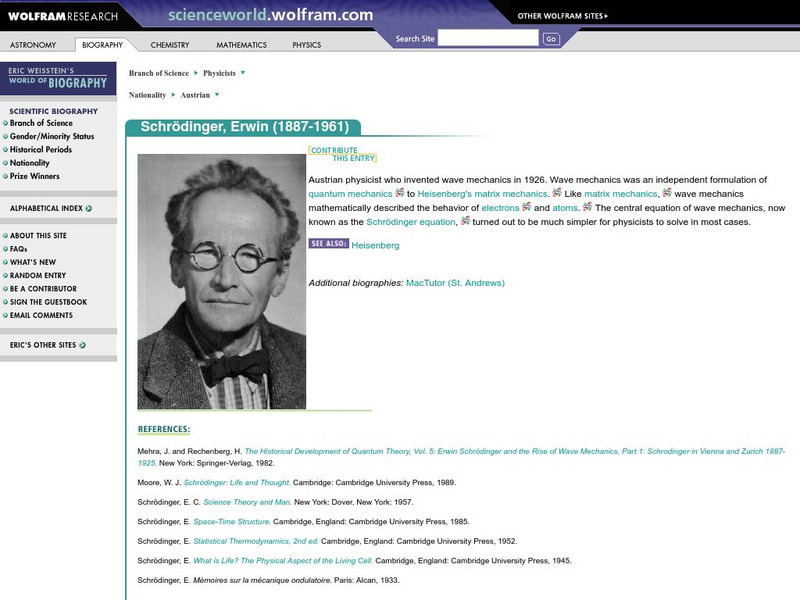
Wolfram Research
Wolfram Science World: Erwin Schrodinger
ScienceWorld.com offers a brief biography and image of Austrian physicist and inventor of wave mechanics, Erwin Schrodinger.
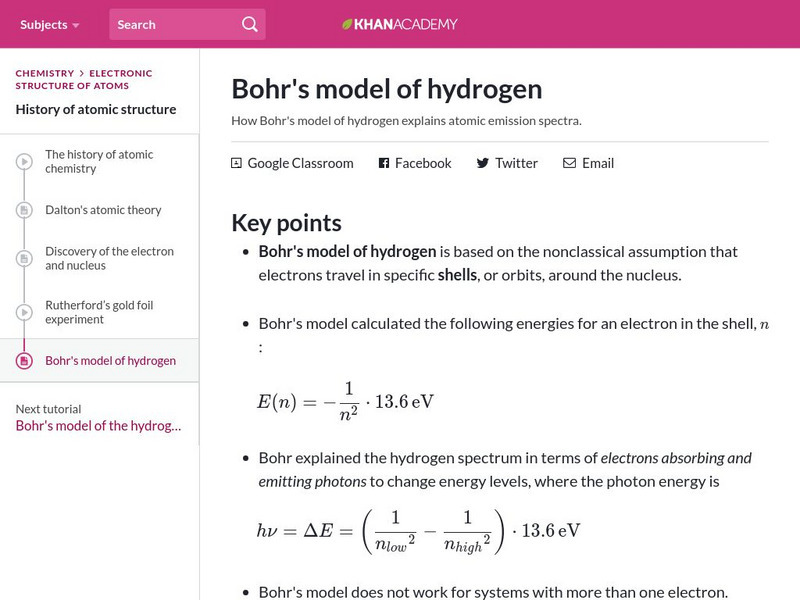
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.