Hi, what do you want to do?
Science Education Resource Center at Carleton College
Serc: Mn Step: Solutions: Solubility and Miscibility
For this activity, students will test the solubility and miscibility of various substances in water, and explain the chemistry involved. They will also be given a solid and a liquid and asked to predict what will happen when each is...
Khan Academy
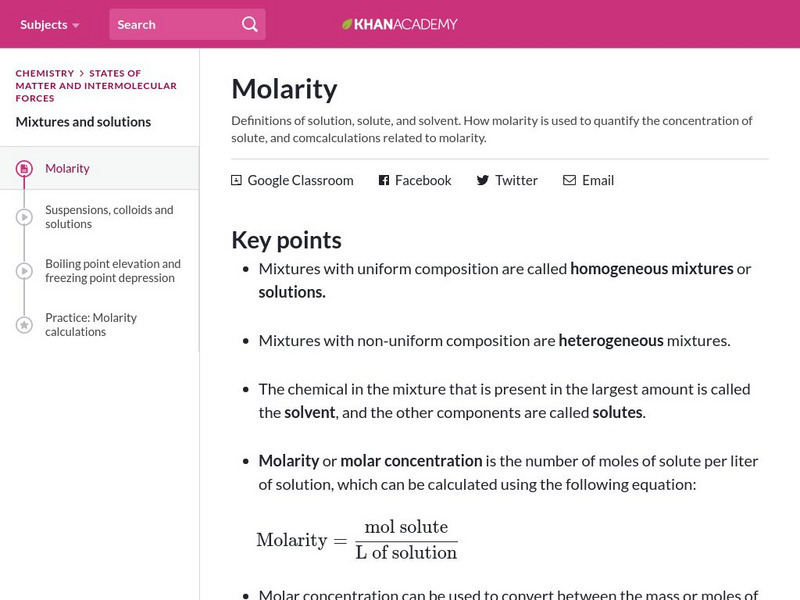
Khan Academy: Molarity
Learn the definitions of a solution, solute, and solvent. Understand how molarity is used to quantify the concentration of solute, and comcalculations related to molarity.
American Chemical Society
Middle School Chemistry: Lesson Plans: Does Temperature Affect Dissolving?
Students identify and control variables to design an experiment to see whether the temperature of a solvent affects the speed at which a solute dissolves.
American Chemical Society
Middle School Chemistry: Lesson Plans: Why Does Water Dissolve Salt?
Students use their own model of a salt crystal and water molecule to show how water dissolves salt. Then, they relate their observations to the structure of salt, water, and alcohol on the molecular level.
TeachEngineering
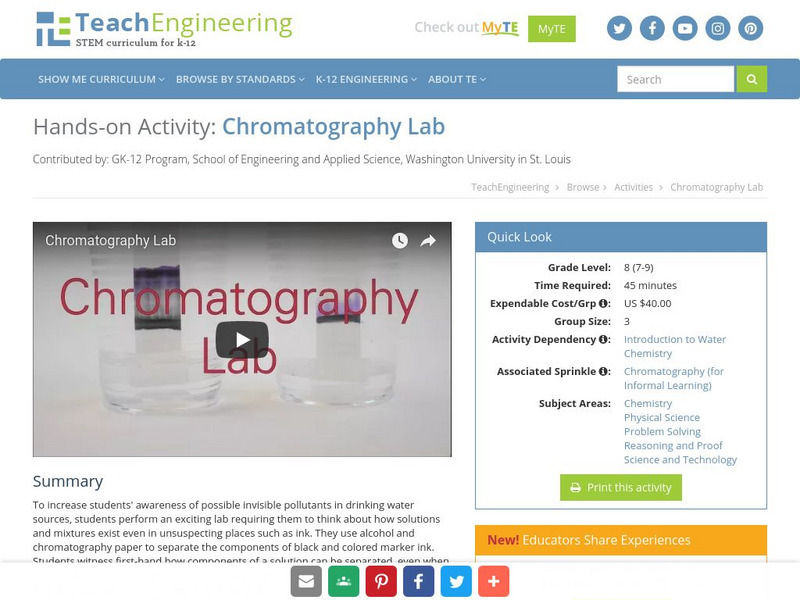
Teach Engineering: Chromatography Lab
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use...
Georgia Department of Education
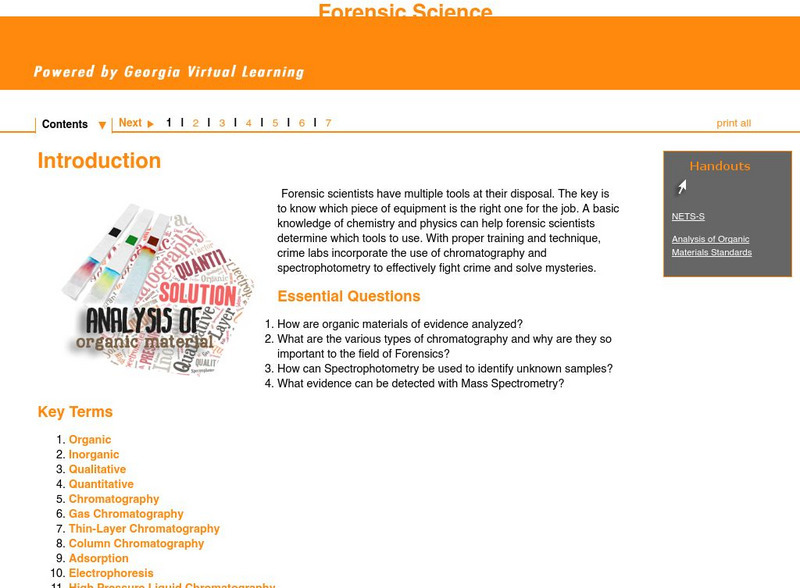
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
Annenberg Foundation
Annenberg Learner: Virtual Particle Lab: Dissolving
Explore what happens when one substance dissolves into another. Run the simulations and see if you can predict the results.
Other
Aqueous
A chemical tutorial describes the term "aqueous" and features examples of how different substances dissolve.
Curated OER
Reverse Osmosis and Osmotic Pressure What They Are
This site gives a good explanation of osmosis and reverse osmosis. Also includes a scientific diagram of the process.
Savvas Learning
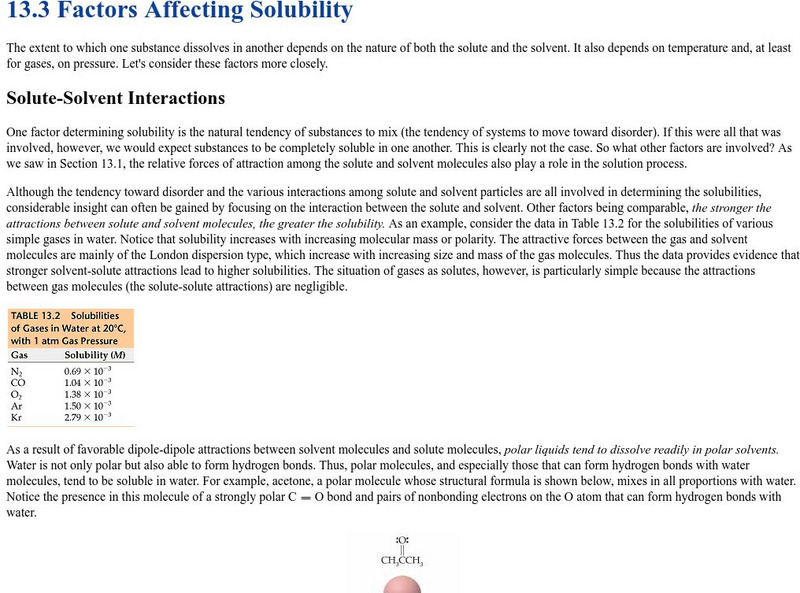
Pearson Education: Factors Affecting Solubility
Learn how the extent to which one substance dissolves in another depends on the nature of both the solute and the solvent.
CK-12 Foundation
Ck 12: Chemistry: Dissolving Process
[Free Registration/Login may be required to access all resource tools.] Describes solution formation and interaction of solute with water.
Science Education Resource Center at Carleton College
Serc: Investigating Chromatography: Selecting Variables
In this lab, students will demonstrate observation skills as they design an experiment to separate colors of various water-based pens in order to learn about mixtures and solutions. Students will determine a variable to test and complete...
Science Education Resource Center at Carleton College
Serc: Mn Step: Spotting Chromatography
A lab activity that introduces chromatography using markers, plus water and acetone as the solvents. Students will take measurements to compare the mobile and stationary phases of the inks, and find the polarity of the inks and solvents....
Sophia Learning
Sophia: Suspensions: Lesson 2
This lesson will explain the properties of a suspension, and describe why it is unique from other mixtures. It is 2 of 2 in the series titled "Suspensions."
Sophia Learning
Sophia: The Properties of Water: Lesson 4
This lesson will provide an understanding of the chemical and physical nature of water. It is 4 of 4 in the series titled "The Properties of Water."
Sophia Learning
Sophia: The Properties of Water: Lesson 1
This lesson will provide an understanding of the chemical and physical nature of water. It is 1 of 4 in the series titled "The Properties of Water."
Other
How to smile.org: Solubility Test
Students can figure out what an unknown crystal is by comparing their dissolving test with the known and unknown crystals. This inquiry activity includes background information and student handout.
Sophia Learning
Sophia: The Properties of Water: Lesson 3
This lesson will provide an understanding of the chemical and physical nature of water. It is 3 of 4 in the series titled "The Properties of Water."
Khan Academy
Khan Academy: Molarity vs. Molality
Learn how molarity and molality differ. The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of a solution is equal to the moles of solute divided by the volume of...