Hi, what do you want to do?
Mocomi & Anibrain Digital Technologies
Mocomi: Difference Between Solution Solute and Solvent
Article offers a comparison between a solution, a solute, and a solution and provides examples.
Other
What Is Life: Aqueous Solutions
Discussion of water as a solvent. Includes material on hydrogen bonds, hydration, hydrophobic effect, acids, basses, and pH.
Savvas Learning
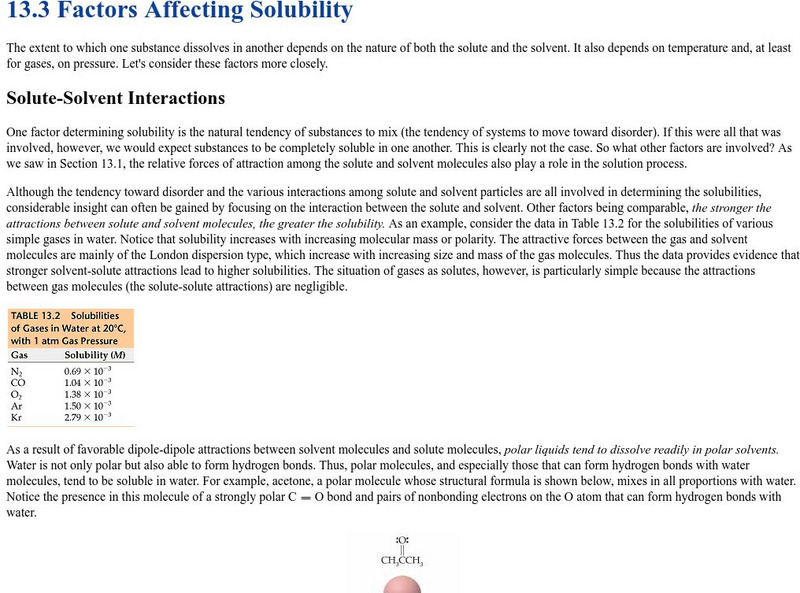
Pearson Education: Factors Affecting Solubility
Learn how the extent to which one substance dissolves in another depends on the nature of both the solute and the solvent.
Utah Education Network
Uen: Finding Solutions
Students will use the terms solute and solvent in describing a solution. They will sketch a solution at the particle level and design and conduct an experiment to determine the factors (e.g., agitation, particle size, temperature)...
Alabama Learning Exchange
Alex: Get in the Mix
Learners will be able to classify a mixture as homogeneous or heterogeneous. Students will be able to create a solution. Learners will be able to identify the solute. Students will be able to identify the solvent. Learners will be able...
CK-12 Foundation
Ck 12: Physical Science: Properties of Solutions
[Free Registration/Login may be required to access all resource tools.] How solutes affect solvents, the freezing point depression and boiling point elevation.
Libre Text
Libre Texts: Chemistry: Solubility and Factors Affecting Solubility
Understand how temperature, pressure, and the presence of other solutes affect the solubility of solutes in solvents.
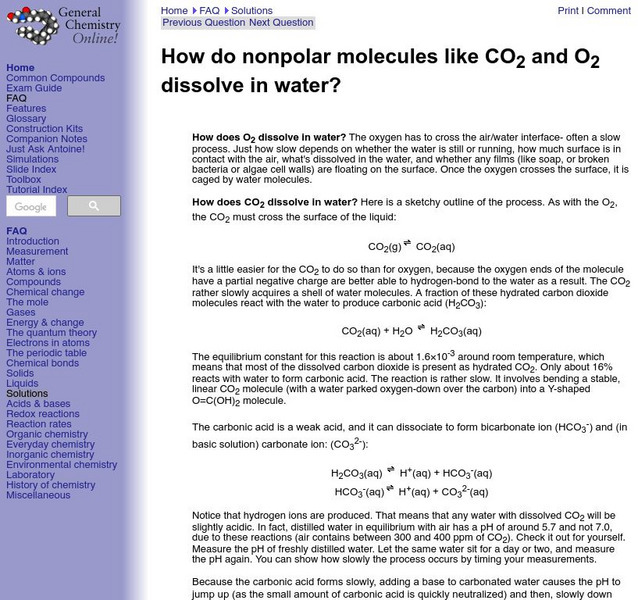
Frostburg State University
University of Frostburg: How Nonpolar Molecules Dissolve
This site from the University of Frostburg provides an explanation of the process by which nonpolar molecules dissolve in water.
Science Struck
Science Struck: The Varied Uses of Chloroform
Describes early uses of chloroform as an anesthetic and the dangers its use posed. Discusses its use as a solvent and some limited uses in dentistry and veterinary medicine. Chloroform was once used in some common products but is now...
San Diego State University
San Diego State University: Polar Solute
A knowledge map with clickable links related to polar solutes.
San Diego State University
San Diego State University: Nonpolar Solute
A knowledge map with clickable links related to nonpolar solutes.
Carnegie Mellon University
Chem Collective: Brownian Motion
Particulate level simulations that show only solute particles are convenient, since they focus student attention on the molecules of most interest. However, such solute molecules move in a Brownian manner. This simulation helps students...
CK-12 Foundation
Ck 12: Osmosis
[Free Registration/Login may be required to access all resource tools.] Tutorial defines osmosis and the difference between osmosis and diffusion. Students will learn how to distinguish solute from solvent and solution and the hypotonic,...
CK-12 Foundation
Ck 12: Colloids and Suspensions
[Free Registration/Login may be required to access all resource tools.] In this lesson, students expand their study of mixtures to show that solids and gases can also act as solvents. Additionally, they take a look at situations in which...
American Chemical Society
Middle School Chemistry: Lesson Plans: Why Does Water Dissolve Salt?
Students use their own model of a salt crystal and water molecule to show how water dissolves salt. Then, they relate their observations to the structure of salt, water, and alcohol on the molecular level.
Georgia Department of Education
Ga Virtual Learning: Physical Science: Solutions Chemistry
Through interactive puzzles, informational text and video clips, students will investigate the properties of solutions.
Vision Learning
Visionlearning: Physical States and Properties: Water: Properties and Behavior
Information relating to the properties of water and its behavior.
US Geological Survey
Usgs: Water Properties and Measurements
Learn about the various attributes of water and how they affect life on Earth.
John Wiley & Sons
Concepts in Biochemistry: Concept Reviews: Water, P H, and Non Covalent Bonding
A detailed review of water and its structure. Included are diagrams, animated graphics, and review quizzes. For the advanced high school student.
CK-12 Foundation
Ck 12: Aqueous Solutions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define a solution and describe the parts of a solution. They will describe how an aqueous solution is formed from both...
Georgia Department of Education
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
TeachEngineering
Teach Engineering: Chromatography Lab
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use...
Annenberg Foundation
Annenberg Learner: Virtual Particle Lab: Dissolving
Explore what happens when one substance dissolves into another. Run the simulations and see if you can predict the results.
Other popular searches
- Solutions Solvent Solute
- Solute Solvent
- Solvent and Solute
- Water Universal Solvent
- Solute and Solvents
- Solute and Solvent Lab
- Organic Solvents
- Solution, Solvent, Solute
- Solvent vs Solute
- Solutes and Solvents
- Universal Solvent
- Water Universal Solvent