Other
Imperial College: Cf Cs
This site from the Imperial College of Science provides a chemistry based discussion of CFCs with formulas and Molecular structures.
Famous Scientists
Famous Scientists: Aage Bohr
Learn about the life of Aage Niels Bohr, and see how his work was pivotal in the development of the theory of the structure of the atomic nucleus.
Sophia Learning
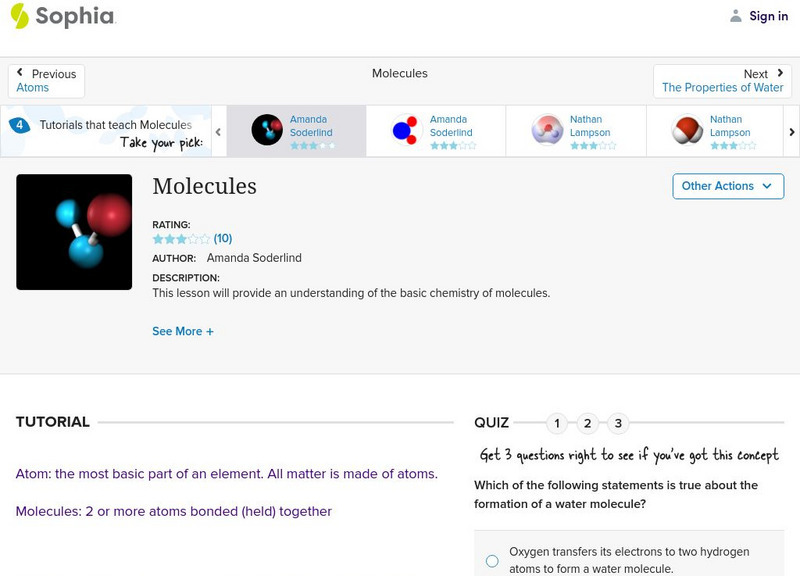
Sophia: Molecules
An audio podcast accompanied by a diagram of a water molecule helps the learner understand molecular structure. [0:23]
Khan Academy
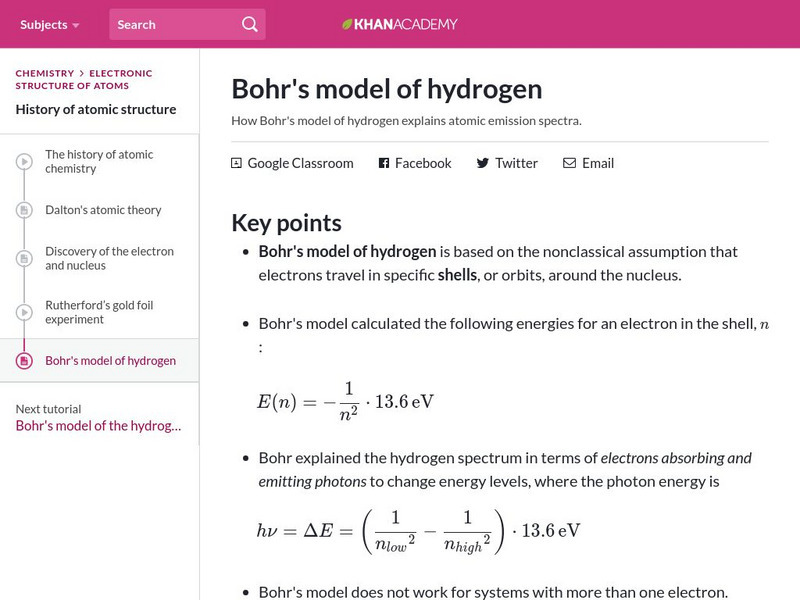
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Rutherford Scattering
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining...
Other
Science Alive: Synthetic vs. Natural: What's the Difference?
Through this reading, students will learn that a substance's properties arise from its molecular structure, not from how it's made (i.e., synthesized by people or found in nature). There is no fundamental difference between natural and...
Concord Consortium
Concord Consortium: How Can a Small Spark Start a Huge Explosion?
In this module Activity 3 investigates When atoms get close to each other, what happens to their potential energy? This activity applies energy concepts to compare the potential energy of atoms that are bonded as molecules with the...
Texas Instruments
Texas Instruments: Atomic Nuclear
This StudyCards stack enables students to review the vocabulary associated with atoms and nuclear chemistry.
Concord Consortium
Concord Consortium: Where Does All the Energy in an Explosion Come From?
Students construct a model of chemical reactions involving energy and electrostatic interactions and compare reactions and changes in energy through the following activities. Activity 1 What energy changes occur during an explosion?...
Concord Consortium
Concord Consortium: What Is Happening When a Spark Occurs?
Students define potential and kinetic energy, explore energy transfer and energy conservation, and connect energy to atomic structure. The following activities guide students to a conclusion: Activity 1. Can my finger start a fire?...
Concord Consortium
Concord Consortium: How Does an Object Become Charged?
Activity 2 in this module examines How do objects become charged? An investigation of where the electrons come from and where they go, when atoms become charged.
Concord Consortium
Concord Consortium: Where Does All the Energy in an Explosion Come From?
In this module Activity 2 investigates What happens to atoms during a chemical reaction? In this activity students explore chemical reactions and develop a model to explain observations of chemical reactions.
Environmental Chemistry
Periodic Table of Elements: Gallium
A very detailed look at the element Gallium, a member of the Boron Group.
Sophia Learning
Sophia: Valence Electrons and Core Electrons
Through a series of slide show presentations and a video lesson, strengthen your understanding of valence electrons. [2:46]
TED Talks
Ted: Ted Ed: What Is the Shape of a Molecule?
George Zaidan and Charles Morton shape our image of molecules. [3:47]
Khan Academy
Khan Academy: Dalton's Atomic Theory
Article explores the key points of Dalton's atomic theory and the laws of conservation of mass and constant composition. Which points do we still use today?
State University of New York
State University of New York: Determining Electron Pair Geometry
The electron-pair geometry of a molecule or ion depends on the number of structurally significant electron pairs in the central atom. Here students are asked to count the number of lone electron pairs and bonded atoms on the central...
Chem Tutor
Chem Tutor: Chemistry: Compounds
This instructional activity focuses on chemical compounds including Ionic and Covalent Bonds, Valences, Lewis Structures, Binary Covalent Compounds, Radicals or Polyatomic Ions, and much more. It also includes a compound worksheet in...
Upper Canada District School Board
Tom Stretton's Chemistry Pages: The Nucleus
This online slide show presents the basic ideas behind the structure of the atomic nucleus.
BBC
Bbc: Gcse Bitesize Science: Different Substances
A short quiz over the general topics of atomic structure of different substances and bonding properties. The quiz gives immediate feedback over with explanation for the correct answer choice.
Kent State University
Kent State: Classification of Matter
Nice tree structure which shows the different separation technique needed for the two sides. Also gives examples of physical changes/separation techniques.
Science Buddies
Science Buddies: Science Careers: Materials Scientist and Engineer
An interest in chemistry and physics is what you need to explore the career of materials scientist or engineer. In this career you manipulate atoms to change the structure of materials to make new products. This Science Buddies site lays...
Science Buddies
Science Buddies: Study Chirality With a Homemade Polarimeter
Some molecules can be either left- or right-"handed." The left- and right-handed molecules have the same number and type of atoms, and their chemical structures look identical, but they are actually mirror images of each other. Many...
OpenStax
Open Stax: Andrew R. Barron: Valence Shell Electron Pair Repulsion (Vsepr) Theory
Detailed explanation of Valence Shell Electron Pair Repulsion (VSEPR) Theory with examples and questions for the reader to check comprehension. Also includes step-by-step instructions for correctly building molecules using VSEPR Theory....
Other popular searches
- Atomic Structure
- Atomic Structure Time Line
- Atomic Structure Crossword
- Atom Structure
- Atomic Structure Lesson Plan
- Atomic Structure Worksheet
- Basic Atomic Structure
- Atomic Structure Bohr Model
- Atomic Structure Interactive
- Chemistry Atomic Structure
- Atomic Structure Lewis Dots
- The Atomic Structure