Hi, what do you want to do?
Stanford University
Stanford Encyclopedia of Philosophy: Democritus
A look at the life of Democritus of Abdera. He helped to develop a theory of atomism, explained in detail here. Other significant ideas he had included his theory of perception, a theory of the soul and its relationship to living things,...
Federation of American Scientists
Fas: National Security Policy Arms Control Disarmament
A lengthy essay describing the negotiations and politics involved in President Kennedy's quest to create a permanent ban on nuclear tests.
Concord Consortium
Concord Consortium: Stem Resources: Excited States and Photons
Students are able to explore the effects of energy on excitation of atoms through simulations, then relate this information to photons at atoms' absorption and emission of light. Multiple-choice and short answer questions are found...
Simon Fraser University
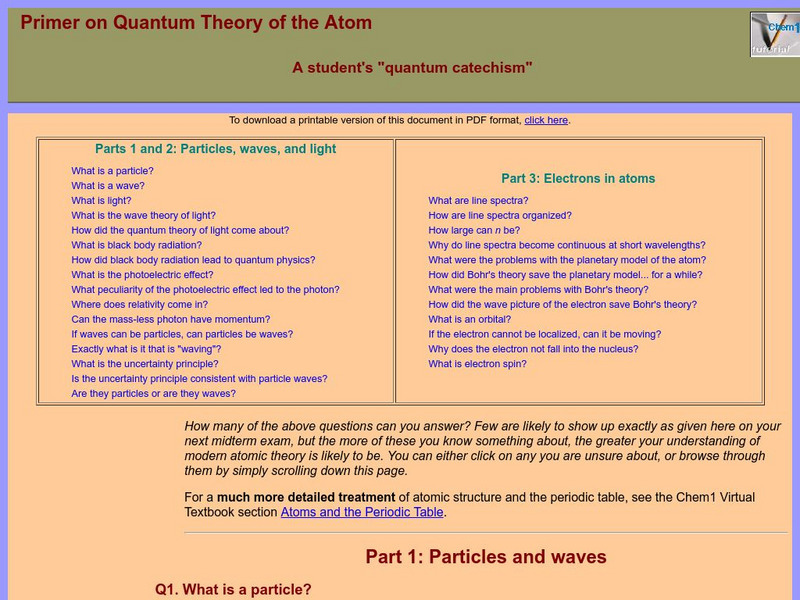
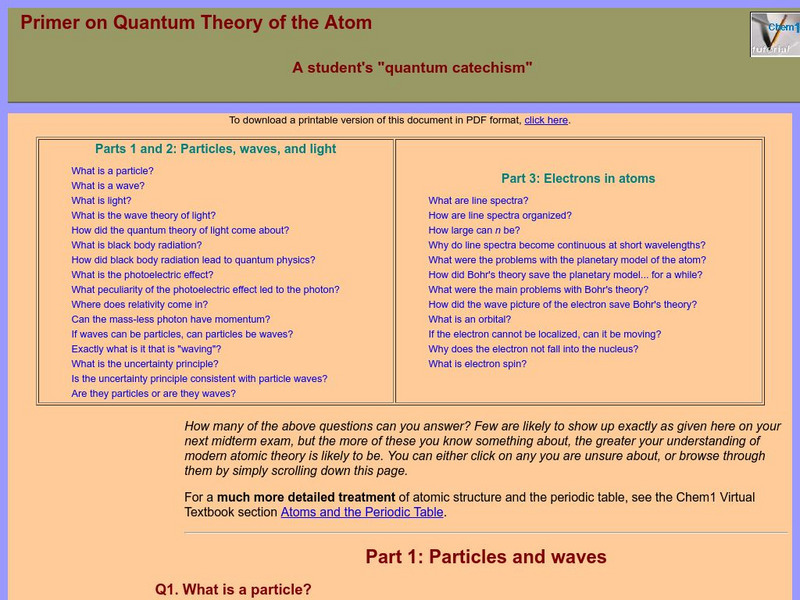
Chem1 Virtual Textbook: What Is an Orbital?
Acting as part of an overview on quantum theory, this section of the site deals with electrons and orbitals. In addition to explain what an orbital is, the site explains the movement of the electron in relation to the nucleus.
University of St. Andrews (UK)
University of St. Andrews: Insulators
The nature of insulators is described at the atomic level. Band gap theory is used to explain what distinguishes insulators from conductors.
PBS
Pbs: People and Discoveries
At this PBS site there is a biography of the Austrian physicist Erwin Schrodinger who lived from 1887 until 1961.
Physics4kids
Physics4 Kids: Modern Physics: Looking Into Atoms
Get an inside look the subatomic particles of atoms.
Encyclopedia Britannica
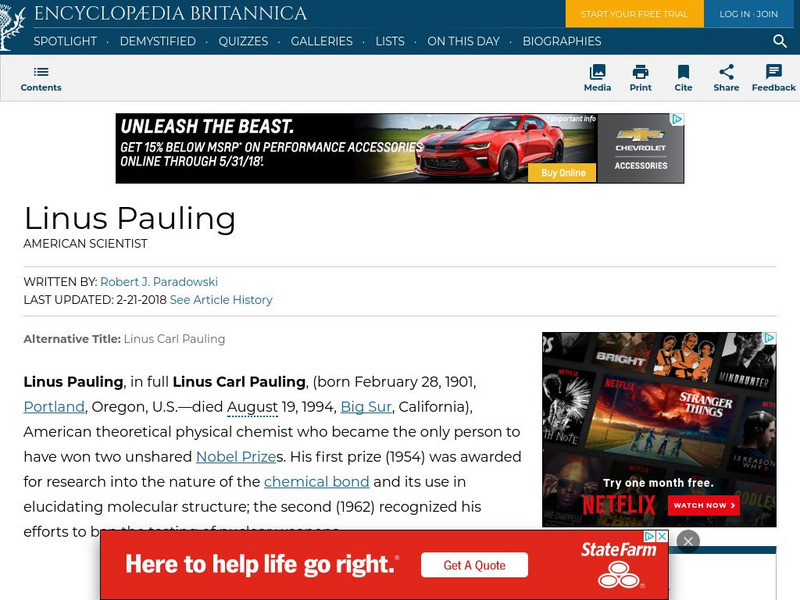
Encyclopedia Britannica: Linus Pauling
This is a brief biography of Linus Pauling, the famous American chemist and two time Nobel Prize winner.
Other
Sir Joseph John Thomson
Have you ever wondered who discovered the electron? The answer is Nobel Prize winning physicist Sir Joseph John Thomson.
Sophia Learning
Sophia: James Chadwick
This lesson explains the discovery of the neutron and Chadwick's experimental process.
Chem4kids
Chem4 Kids: Atoms: Orbitals
The website provides an explanation of the electrons' position in their orbitals as they spin around the nucleus. Quantum theory is also explored by explaining that the exact location of a specific electron is a not exact but a guess.
National High Magnetic Field Laboratory
Magnet Academy: Edward Purcell
Edward Mills Purcell was an American physicist who received half of the 1952 Nobel Prize for Physics for his development of a new method of ascertaining the magnetic properties of atomic nuclei. Known as nuclear magnetic resonance...
University of California
Ucla: Maria Goeppert Mayer
UCLA Physics presents three documents here on Maria Goeppert Mayer, including a brief biography, a longer biography, and a physics meeting address on Mayer and the specifics of her work in nuclear physics.
Towson University
Towson University: Shapes of Molecules
This chemistry class printout details the main points of molecular geometry and explains bond hybridization and bond angles.
Wikimedia
Wikipedia: Mutual Assured Destruction
A complete description of the concept of Mutual Assured Destruction. Explains what the concept means, and how the concept was used during the Cold War and up to the George W. Bush Administration. Includes links to terms that may require...
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
University of Maryland
University of Maryland: Optics, Electromagnetic Waves
This site from the University of Maryland provides part of an anecdotal history of optics and the study of light. Extremely thorough treatment of how scientists came to believe in the wave nature of light, the idea of an electromagnetic...
Simon Fraser University
Chem1 Virtual Textbook: What Is Light?
Acting as part of an overview on quantum theory, this section of the site answers the question, what is light? In answering the question, specific discussion is directed toward light waves and theories of light.
Simon Fraser University
Chem1 Virtual Textbook: Electrons
Acting as part of an overview on quantum theory, this section of the site deals with questions related to electrons, such as "If the electron cannot be localized, can it be moving?" and "Why does the electron not fall into the nucleus?"
Mocomi & Anibrain Digital Technologies
Mocomi: Albert Einstein Biography
Useful biographical information, inventions, and interesting facts about German-born physicist, Albert Einstein, known for developing the theory of relativity.
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...