Hi, what do you want to do?
Simon Fraser University
Chem1 Virtual Textbook: The Quantum Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site specifically addresses the quantum atom and related topics. The related topics include the wave function and its physical...
National Museum of Science and Industry (UK)
Ingenious: Atomic and Nuclear Physics Images
This series of images is a combined collection of over three museums' collections of atomic and nuclear physics images. The images are sorted by pages with about nine images per page and three pages.
Other
Epicurus: Intro to Lucretius: On the Nature of Things
Detailed article on Lucretius, who believed that the soul is not a distinct, immaterial entity but a chance combination of atoms that does not survive the body.
Nobel Media AB
The Nobel Prize: Max Planck Biographical
A biography of Max Planck, covering his research interests and contributions to science, as well as more personal information about his family and life.
University of Wisconsin
The Why Files: Learning About Neutrinos
A learning resource to understand what neutrinos are and their importance to science.
Crescent Public Schools
The Internet Science Room: Quantum Numbers
Using diagrams, illustrated examples, and student practice this chemistry class tutorial explains quantum numbers.
Other
Indiana University Northwest: The Aufbau Principle
The Aufbau Principle defined. Site contains charts, graphs, and examples to help in your learning of the Principle and its many purposes.
CK-12 Foundation
Ck 12: Chemistry: Bohr's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Explains the basic principles of the Bohr hydrogen atom.
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Theory
Acting as part of an overview on quantum theory, this section of the site answers the question, "How did Bohr's theory save the planetary model, for a while?" A section below also discusses the primary problems with Bohr's theory.
Khan Academy
Khan Academy: Applications of Hard Soft Acid Base Theory
Questions related to the electronic structure and atomic chemical behavior of acid and bases.
Frostburg State University
General Chemistry Online: History of Chemistry
A compilation of frequently asked questions in the world of chemistry. Look for answers about atomic theory, periodic table, and Lavoisier in this FAQ.
Frostburg State University
General Chemistry Online: Atoms, Elements, and Ions Faq
Get the skinny on all things frequently asked about isotopes, subatomic particles and atomic theory. Find out what a "dalton" is or the difference between Na+ and Na.
Other
Le Moyne University: John Dalton
This site from the Le Moyne University provides excerpts from Dalton's "A New System of Chemical Philosophy" published in 1808. Includes Dalton's table of atomic weights and and scanned atomic symbols.
Other
Particle Adventure Dutch Version
Dutch version of the well-known "Particle Adventure" physics website that teaches students about atoms, mass, particle physics, and quantum physics. The site discusses theories related to physics and provides other links related to the...
CK-12 Foundation
Ck 12: Chemistry Simulation: Rutherford's Gold Foil Experiment
[Free Registration/Login Required] How can we predict an atom's structure, if we cannot see an atom? Using the Rutherford's Gold Foil Experiment, make your own model and test out the model.
Other
Chemical Heritage Foundation: John Dalton
This interesting site has a biography of John Dalton as well as other influential scientists in the field of chemistry.
Wikimedia
Wikipedia: Max Planck
Discover the life and accomplishments of the great German scientist Max Planck. This site also provides links to sites explaining theories and scientific terms that are associated with Planck.
University of Houston
University of Houston: Engines of Our Ingenuity: John Dalton's Notation
This is part of a small radio show at the University of Houston. It talks about how John Dalton came up with his version of chemical notation, and how it differs from our version of it today. It is available in audio form also.
Soylent Communications
John Dalton
A very thorough biography of John Dalton on this site made by Notable Names Database. Also gives the names of books that were written about him or his ideas.
National Institute of Standards and Technology (NIST)
Nist: Bose Einstein Condensation in Dilute Atomic Vapor
Gives a brief introduction to Bose-Einstein Condensation (BEC) and how it was demonstrated at a laboratory at the NIST.
Lawrence Berkeley National Laboratory
Berkeley Lab: Particle Adventure: The Standard Model
An introduction to the Standard Model, a theory which attempts to explain atomic structure using leptons, quarks, and force carrier particles.
Other
Ancient Greek Philosophy: Democritus of Abdera
A discussion of the theories of Democritus (c. 460-370 BC). He is best known as an atomist, having developed an atomic theory. Also discusses the ideas of other philosophers who are believed to have influenced Democritus.
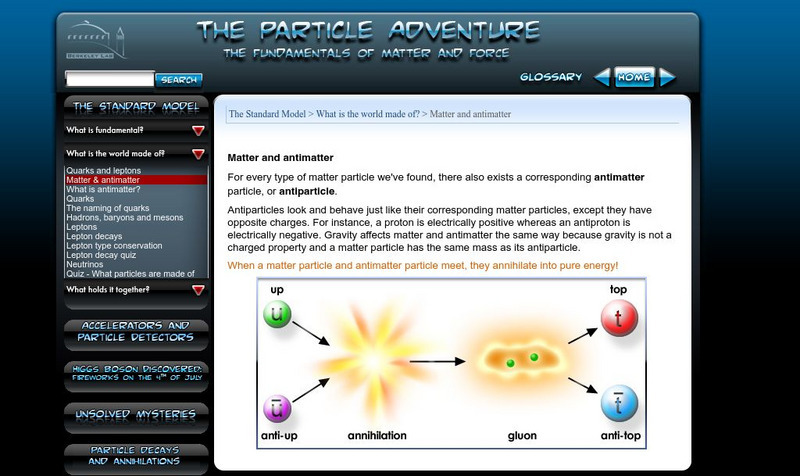
Lawrence Berkeley National Laboratory
Berkeley Lab: Particle Adventure: Matter and Antimatter
The beginning of an informative tutorial on antimatter, covering quarks, hadrons, baryons, mesons, leptons, and neutrinos.
Other
Ippog: Hands on Particle Physics
Looking for more insight into particle physics? Search among several general particle physics resources. Or locate a specific particle physics institute in your area of the world.