Hi, what do you want to do?
National Institute of Educational Technologies and Teacher Training (Spain)
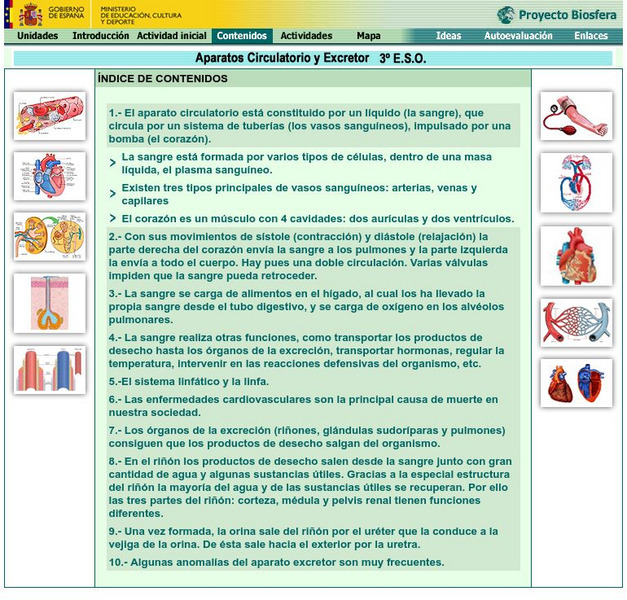
Ministerio De Educacion: Aparatos Circulatorio Y Excretor
This unit explores two important systems related to the functions of nutrition: the circulatory and excretory system. It contains 9 interactive activities.
Khan Academy
Khan Academy: Molarity, Molality, Osmolarity, Osmolality, and Tonicity What's the Difference?
See how each of these terms tells us something different about a solution.
OpenSciEd
Open Sci Ed: 7.4 Matter Cycling & Photosynthesis
Where does food come from and where does it go next? This unit helps students figure out that they can trace all food back to plants, including processed and synthetic food. They obtain and communicate information to explain how matter...
Khan Academy
Khan Academy: Molarity vs.osmolarity
Molarity and osmolarity are two distinct concepts. Molarity (M) is the number of moles of solute per liter of solution. The unit of molarity is the mole (mol). Osmolarity (Osm/L) is the total concentration of all solutes in the solution....
Khan Academy
Khan Academy: Introduction to Lab Values and Normal Ranges
An explanation of lab values following a blood test using a universal model. Also discussed are reasons why a number may be outside of a normal range. These variances include: age, gender, individual laboratory techniques, and the given...
Khan Academy
Khan Academy: What Is Equivalent?
The equivalent is the amount of a substance which will either react or supply with one mole of hydrogen ions and acid-base reactions or do the same with one mole of electrons in a redox reaction.
Khan Academy
Khan Academy: What's Inside Blood?
An explanation of blood from the time it is drawn from the arm to its centrifugation to an analysis of the three components of plasma, white blood cells, and red blood cells.
Khan Academy
Khan Academy: Molarity vs. Molality
Learn how molarity and molality differ. The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of a solution is equal to the moles of solute divided by the volume of...