National Institute of Open Schooling
Periodic Table and Atomic Properties
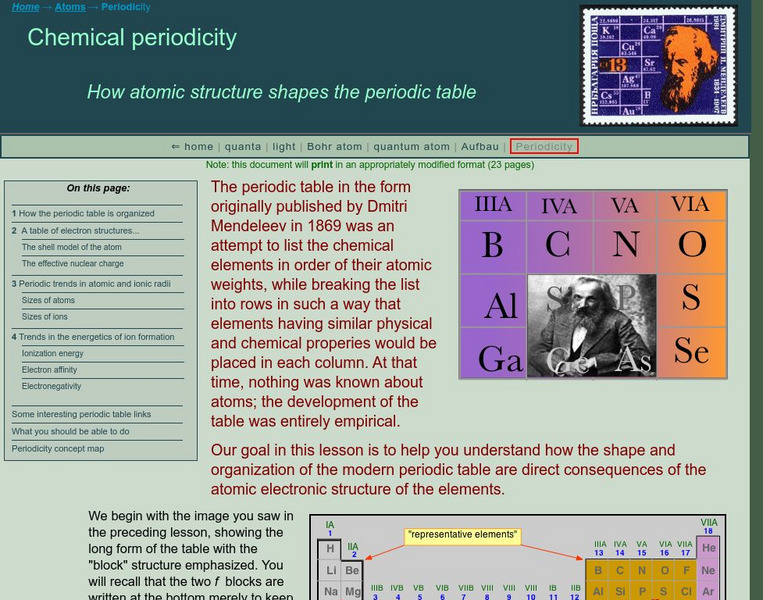
An in-depth lesson, the fourth activity in a series of 36, begins with teaching how the periodic table's arrangement came to its current design. Using this knowledge, pupils then move on to analyze the arrangement of elements to their...
National Institute of Open Schooling
General Characteristics of the p-Block Elements
The 20th installment in a series of 36 focuses on the characteristics of the p-block elements. Learners discuss, read about, and answer questions pertaining to the occurrence of these elements in nature, their electron configurations,...
National Institute of Open Schooling
Hydrogen and s-Block Elements
Lesson 19 in the series of 36 analyzes the element hydrogen and the s-block elements. Through readings, answering questions, and discussion, learners write about and explain their occurrence, physical and chemical properties, and...
Simon Fraser University
Chem1 Virtual Textbook: Periodic Trends in Ion Formation
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses electron affinity and ionization energy in relation to ion formation. Charts and graphs are included as well.
Oklahoma State University
Oklahoma State University: Ionization Energy Trends
Site that discusses trends in ionization energy across and down the periodic table. Apparent contradictions are explained using electron configurations.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
Wikimedia
Wikipedia: Electronegativity
This encyclopedia article from Wikipedia gives a definition for what electronegativity is, information on the two scales of electronegativity that are in common use (Pauling and Mulliken Scales), and a discussion on electronegativity...