Curated OER
Solving Saturn's Mysteries
Middle schoolers study the Cassini spacecraft and its travel to Saturn. They discover the results of the space mission and examine images of Saturn that Cassini sent back.
Curated OER
i-Density Crisis
Eighth graders determine the density using mass and volume. In this science lesson, 8th graders explain why some materials float or sink. They estimate the density of objects based on whether it floats or sinks in a liquid of known...
CK-12 Foundation
Ck 12: Water Properties
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will describe the structure and polarity of a water molecule. They will also describe the hydrogen bonding that occurs in...
New York University
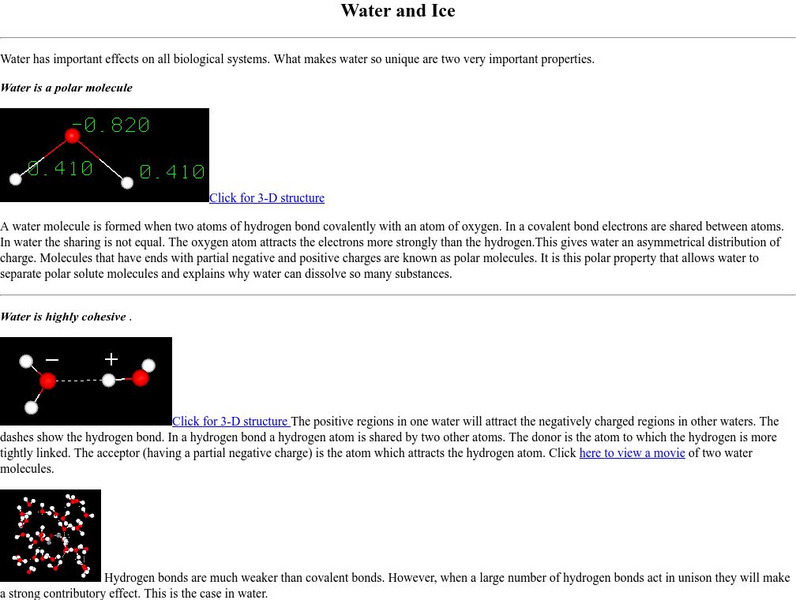
New York University: About Water and Ice
Page uses movies and 3D images to explain how properties of water relate to polarity and hydrogen bonding.
CK-12 Foundation
Ck 12: Properties of Water
[Free Registration/Login may be required to access all resource tools.] In this learning module, students will investigate the chemical and physical properties of the unique and important substance, water.
Science Buddies
Science Buddies: Can Water Float on Water?
Of course it can, you say: ice is water and ice floats. And you're right. But we're talking about water in the liquid phase Can liquid water float on water? The goal of this project is to investigate what happens to layers of water with...
Other
Lunar and Planetary Institute: Explore! Ice Worlds
Features a collection of hands-on activities, investigations, and explorations designed to engage students in learning about ice, both in the solar system and on planet Earth.
American Chemical Society
American Chemical Society: Explore Chemistry: Water and Chemistry
Explore water's unique properties in the activities here. Includes ideas for starting a chemistry club, ideas for Earth Day, articles, and videos.
TED Talks
Ted: Ted Ed: Why Does Ice Float in Water?
Ever wonder why solid ice floats in liquid water? In this video lecture you will explore the special properties of water. Learn "the science behind how hydrogen bonds keep the ice in your glass (and the polar ice caps) afloat". At the...
CK-12 Foundation
Ck 12: Chemistry: Structure of Ice
[Free Registration/Login may be required to access all resource tools.] Covers structure of ice and hydrogen bonding in ice.
University Corporation for Atmospheric Research
Ucar: Just a Phase: Water as a Solid, Liquid, and Gas
This site helps students construct a model of the arrangement of water molecules when present as solid, liquid or gas. Includes background information, lesson plans, links to standards and assessment ideas.
Climate Literacy
Clean: What Happens to Ice in Water?
Students investigate the properties of water in the ice and liquid phase as it relates to convection in the ocean and density driven circulation, and ultimately the climate.
Massachusetts Institute of Technology
Mit: Blossoms: Will an Ice Cube Melt Faster in Freshwater or Saltwater?
Engage young scholars in the study of the ocean and saltwater with these activities. Students will see that saltwater has different physical properties than freshwater - mainly density. This instructional activity can serve as a...
American Chemical Society
American Chemical Society: Best of Wonder Science: Ice of a Different Color [Pdf]
An experiment to test what happens to water when salt or sugar is added and it is then frozen into ice cubes. Students also explore the ice's physical properties by rubbing cubes on sandpaper and dropping a heavy object on each type.
University of California
University of California: Seawater Density & Salinity [Pdf]
Describes the properties of seawater and the variations depending on its location, e.g., near a shoreline, in an estuary, or as sea ice. Discusses the instruments scientists use to measure the density of water and explains other...
TeachEngineering
Teach Engineering: How Cold Can You Go?
Students explore materials engineering by modifying the material properties of water. Specifically, they use salt to lower the freezing point of water and test it by making ice cream. Using either a simple thermometer or a mechatronic...
Other
Science House: Ice Cream
Experiment shows students how to use the lowered freezing point of water to chill another mixture (ice cream) to the solid state. Teacher's notes provide background information.
Science Buddies
Science Buddies: Are You Gellin'?
Chances are, you have several materials around your house made of gelatinized materials. Gels are used in all kinds of products and materials: pudding, diapers, insoles, packaging, ice cream, toothpaste, and much more. In this project,...
TeachEngineering
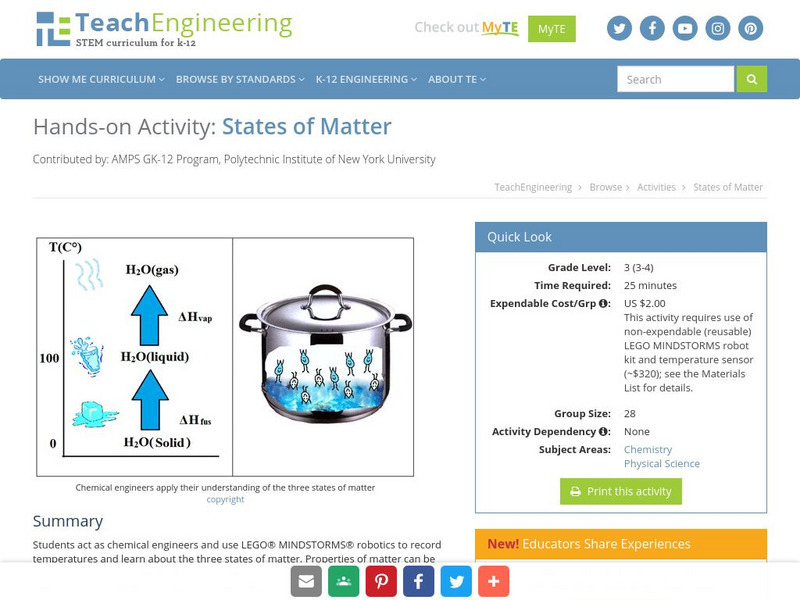
Teach Engineering: States of Matter
Students act as chemical engineers and use LEGO MINDSTORMS NXT robotics to record temperatures and learn about the three states of matter. Properties of matter can be measured in various ways, including volume, mass, density and...
University of Chicago
Center for Astrophysical Research in Antarctica: Don't Be Too Flaky
After reading some facts about the properties of water, students conduct experiments to measure the relative densities of water, ice and snow and submit the results to the website.
Other
Science Alive: Melting Point Simulation
Percy Julian and Josef Pikl used the fact that melting point-the temperature at which a substance changes from a solid to a liquid-is a characteristic property of a substance to prove that the British chemist Robert Robinson could not...