Curated OER
Demonstrating That Light is Dissipated as Chlorophyll a Fluoresces
Students examine the concepts of light and chlorophyll. They participate in an experiment in which the chlorophyll shows as a red ring on the test tube. They answer discussion questions to end the lesson plan.
Curated OER
Sea Ice Research
Students study sea ice and its importance in climate and climate change. They discuss sea ice as a presence of a food source for marine animals in the arctic and complete a lab activity. After completing the lab, they watch a video...
State University of New York
State University of New York: Atomic Absorption and Emission
This module simulates the excitation of hydrogen atoms through irradiation with electromagnetic radiation of different wavelengths.
Friesian School
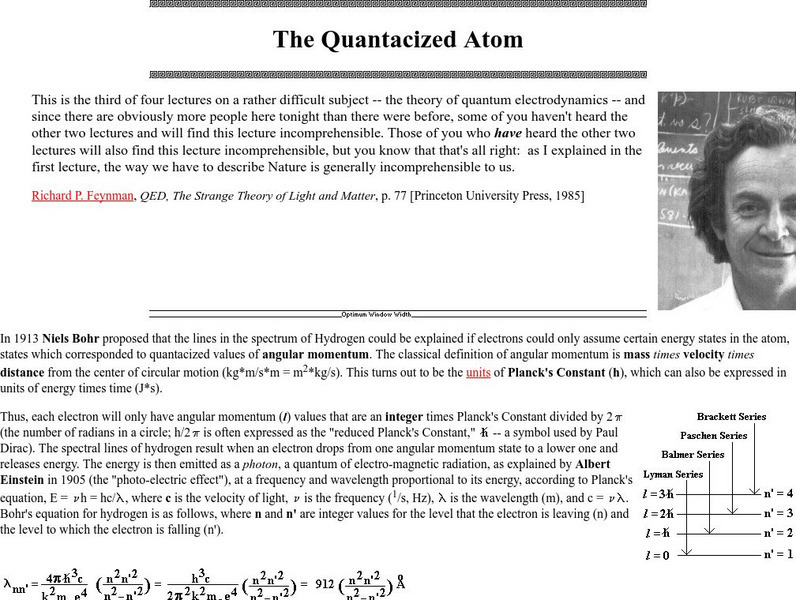
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
PBS
Pbs Learning Media: What Is Albedo?
When light strikes an object it can cause reflection or absorption depending on the wavelength of the light and the property of the material that makes up the object. Watch this whiteboard animation for a full explanation and to find out...
TeachEngineering
Teach Engineering: Exploring Light: Absorb, Reflect, Transmit or Refract?
In a hands-on way, students explore light's properties of absorption, reflection, transmission and refraction through various experimental stations within the classroom. To understand absorption, reflection and transmission, they shine...
University of Colorado
University of Colorado: Physics 2000: Balmer's Formula
A short description of a physics lab involving the determination of the wavelength of the four spectral lines in the hydrogen emission spectrum. Perhaps the most important part of the page is the picture of the spectrometer.
CK-12 Foundation
Ck 12: Chemistry Simulation: Neon Lights
[Free Registration/Login Required] Neon lights are a type of discharge tube. Observe how electrons create colored light in a hydrogen gas discharge tube. Can you figure out why hydrogen's emission spectrum contains more than one color of...
Dartmouth College
Dartmouth College: Chem Lab: Scanning Spectrometer
The scanning spectrometer measures absorbance vs. wavelength for liquid samples. Directions on how to use this instrument are provided here.
University Corporation for Atmospheric Research
Ucar: Atmospheric Processes Radiation
This site provides background information, images, and an activity to help students understand the concept of radiation. Includes both the student pages and a teachers guide with lesson plan.
Oklahoma Mesonet
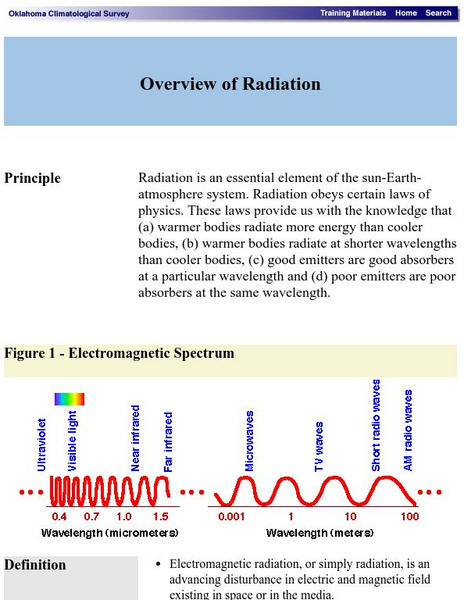
Oklahoma Climatological Survey: Overview of Radiation
This site details what radiation is, the physics of radiation, and radiative transfer as it occurs in nature. Content explores the electromagnetic spectrum, electromagnetic waves, properties of radiation, and solar radiation.
University of California
Center Science Edu.: Electromagnetic Radiation on Trial
Here is a 1-5 day unit on electromagnetic radiation that features a teacher guide and student activities with extensions.