Hi, what do you want to do?
Khan Academy
Khan Academy: Density and Pressure: Specific Gravity
A video exploring what specific gravity is and how to calculate it for an object. Learn how specific gravity can tell you whether an object will float or sink in a fluid. Also understand how it also can be used to determine the amount of...
Khan Academy
Khan Academy: Fluids: Archimedes Principle and Buoyant Force
Learn about the existence of an upward force on an object equal to the weight of the fluid displaced while that object is submerged in a fluid with this video. This video will introduce students to Archimedes' principle and buoyant...
Khan Academy
Khan Academy: Fluid Dynamics: Volume Flow Rate and Equation of Continuity
This video lecture shows how the equation of continuity is derived for a dynamic fluid. [9:59]
Khan Academy
Khan Academy: Thermodynamics Part 1 : Molecular Theory of Gases
A video explaining how intuition can be used to explore the molecular theory of gases. Video explains that kinetic energy of the molecules of gas is proportional to the volume multiplies by the pressure. [9:49]
Khan Academy
Khan Academy: Specific Heat and Latent Heat of Fusion and Vaporization
A video lecture explaining specific heat, heat of fusion, and the heat of vaporization. See a step-by-step example of how to calculate the amount of heat needed to change the temperature of water. Also explored is the energy required to...
Khan Academy
Khan Academy: Law of Thermodynamics: Macrostates and Microstates
Learn that macrostates properties include macroscopic properties such as: pressure, temperature, and volume while microstates includes microscopic properties like: kinetic energy, force, and velocity with this video. Video will also...
Khan Academy
Khan Academy: Circuits: Capacitors and Capacitance
Get an inside look at a capacitor in this video to see how capacitors store charge. Also given is several examples of how to use the formula for capacitance to calculate charge, voltage, and capacitance. [5:42]
Khan Academy
Khan Academy: Mechanical Waves and Sound: Introduction to Mechanical Waves
This video lecture will introduce the concepts of transverse and longitudinal waves. [13:02]
Khan Academy
Khan Academy: Production of Sound
A video explaining how a speaker produces sound by transporting energy through the medium. [3:45]
Khan Academy
Khan Academy: Mechanical Waves and Sound: Standing Waves
A video exploring standing waves. [14:18]
Khan Academy
Khan Academy: Introduction to the Doppler Effect
Do you know why a siren has a high-pitched sound as it moves towards you but when it moves away it has a low-pitch sound? In this video learn about how the Doppler Effect causes this perceived change in the pitch of a sound. [10:36]
Khan Academy
Khan Academy: Specular and Diffuse Reflection
A video focusing on understanding the two types of reflection: specular and diffuse. Video discusses what causes these types of reflection as well as gives examples. [10:59]
Khan Academy
Khan Academy: Electromagnetic Waves and the Electromagnetic Spectrum
Learn about the coupling of an electric field with a magnetic field to create electromagnetic waves in this video. Video also discusses how different types of electromagnetic waves have different wavelengths which forms the...
Khan Academy
Khan Academy: Elements and Atoms
A video lecture that discusses how elements relate to atoms. Also discussed are protons, electrons, and neutrons. [13:08]
Khan Academy
Khan Academy: Heisenberg Uncertainty Principle
A video showing the mathematical description of the uncertainty principle to associate it with the momentum of electrons to solve for the position of the electron. [10:17]
Khan Academy
Khan Academy: Heisenberg Uncertainty Principle
An explanation of Heisenberg's Uncertainty Principle. [10:18]
Khan Academy
Khan Academy: Orbitals
A video introducing orbitals and how electrons "get" to orbital levels. [13:37]
Khan Academy
Khan Academy: Dot Structures I: Single Bonds
A video introducing students at how to draw dot structures for simple organic molecules that have a single bond. Video gives step-by-step instructions with examples. [6:56]
Khan Academy
Khan Academy: Structure and Bonding: Sp3 Hybridized Orbitals and Sigma Bonds
Learn that a sp3 hybridized orbital is a combination of s and p orbital in this video. Also see an example of when a hybridized orbital exist by looking at when carbon forms a sigma bond. [16:22]
Khan Academy
Khan Academy: Structure and Bonding: Condensed Structures
In this video learn how to take a Lewis dot structure to a partially condensed structure to a condensed structure. Video gives an example of this process. [6:48]
Khan Academy
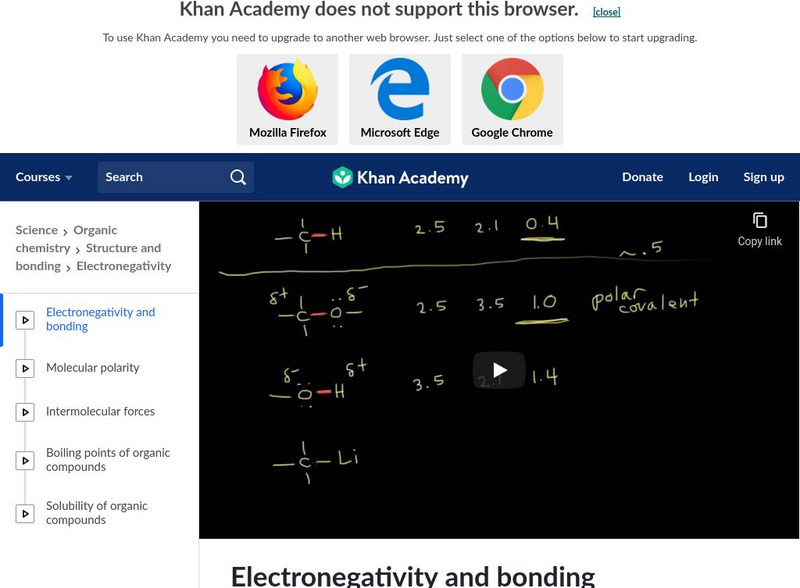
Khan Academy: Structure and Bonding: Electronegativity and Bonding
Learn how to classify bonds as covalent, polar covalent or ionic by using the differences in electronegativity in this video. Video explains the Pauling scale to find the electronegativity differences in bonding. [11:38]
Khan Academy
Khan Academy: Comparing Formal Charges to Oxidation States
This video will show you how to count electrons by understanding formal charges and oxidation states. [5:45]
Khan Academy
Khan Academy: Acid Base Definitions
In this video, learn the definitions of acid and base by studying Bonsted-Lowry reaction theory and Lewis Acid-Base concept. Understand what a proton donor and proton acceptor are in this video. [8:30]
Khan Academy
Khan Academy: Representing Structures of Organic Molecules
A video discussing the notation and nomenclature of organic molecules. [7:28]