Curated by
ACT
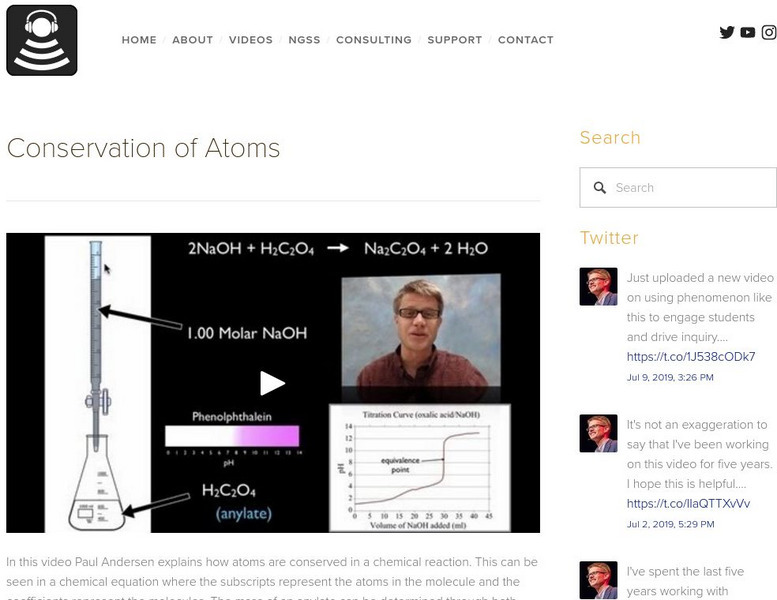
Paul Andersen explains how atoms are conserved in a chemical reaction. This can be seen in a chemical equation where the subscripts represent the atoms in the molecule and the coefficients represent the molecules. The mass of an anylate can be determined through both gravimetric analysis and a titration. [12:18]
3 Views
0 Downloads
Concepts
Additional Tags
Classroom Considerations
- Knovation Readability Score: 5 (1 low difficulty, 5 high difficulty)