Curated by
ACT
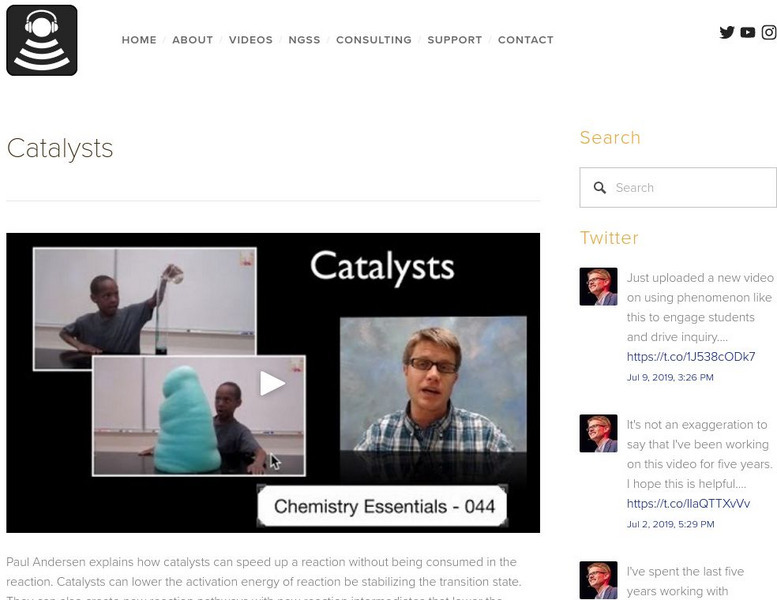
Paul Andersen explains how catalysts can speed up a reaction without being consumed in the reaction. Catalysts can lower the activation energy of reaction be stabilizing the transition state. They can also create new reaction pathways with new reaction intermediates that lower the overall activation energy. See the additional concept map and slideshow. [4:09]
3 Views
0 Downloads
Concepts
Additional Tags
Classroom Considerations
- Knovation Readability Score: 5 (1 low difficulty, 5 high difficulty)