Curated by
ACT
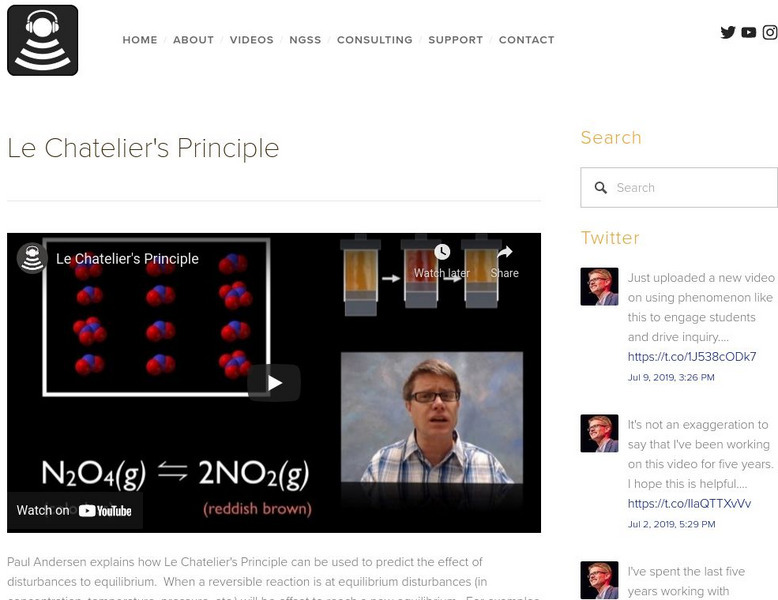
In the following video Paul Andersen explains how Le Chatelier's Principle can be used to predict the effect of disturbances to equilibrium. When a reversible reaction is at equilibrium disturbances will be offset to reach a new equilibrium. For examples when more reactants are added the reaction will move to the right to reestablish the equilibrium constant. [7:01]
3 Views
0 Downloads