Curated by
ACT
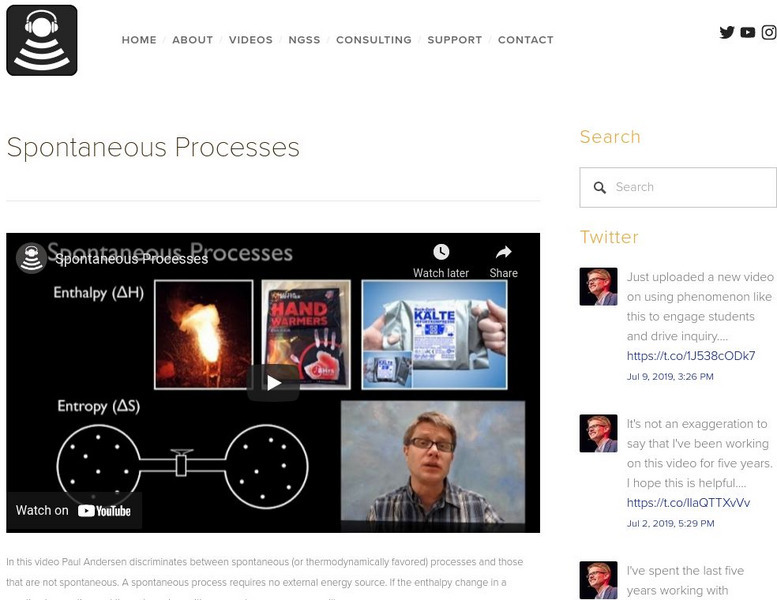
In this video, Paul Andersen discriminates between spontaneous (or thermodynamically favored) processes and those that are not spontaneous. A spontaneous process requires no external energy source. If the enthalpy change in a reaction is negative and the entropy is positive a spontaneous process will occur. Be sure to explore the additional resources provided. [7:43]
3 Views
0 Downloads
Concepts
Additional Tags
Classroom Considerations
- Knovation Readability Score: 4 (1 low difficulty, 5 high difficulty)