Curated by
ACT
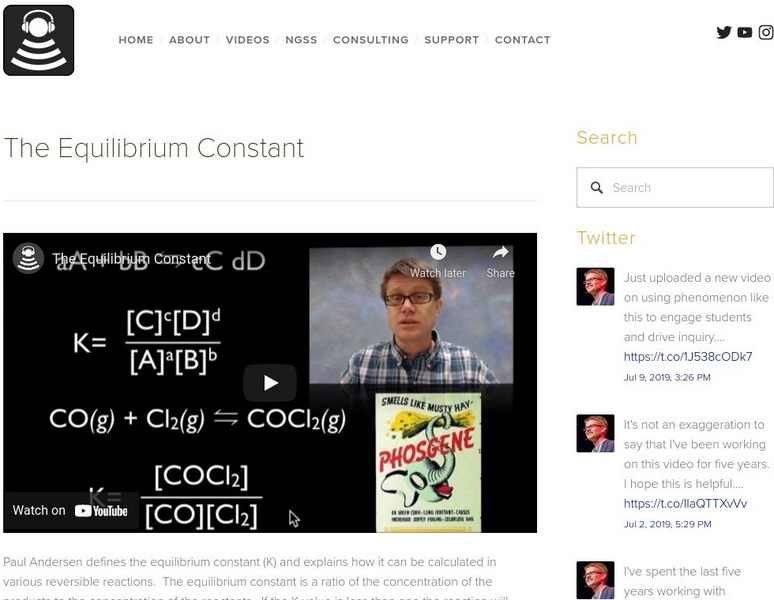
In the following video Paul Andersen defines the equilibrium constant (K) and explains how it can be calculated in various reversible reactions. The equilibrium constant is a ratio of the concentration of the products to the concentration of the reactants. If the K value is less than one the reaction will move to the left and if the K value is greater than one the reaction will move to the right. [6:15]
3 Views
0 Downloads
Additional Tags
Classroom Considerations
- Knovation Readability Score: 5 (1 low difficulty, 5 high difficulty)