Curated OER
2009 U. S. National Chemistry Olympiad - Local Section Exam
Here is a copy of a past national challenge exam that you can use in your general chemistry course as a unit or semester review. Sixty multiple-choice questions query learners on properties of matter, stoichiometry, reactions, and...
Beyond Benign
Green Chemistry, Biomimicry and Intermolecular Forces
Did you know plywood was invented around 3500 B.C.? It was also featured as something new and unusual at the 1905 World's Fair. Scholars complete an experiment with various types of adhesive. Then they read three case studies and answer...
K20 LEARN
The Attraction is REAL
How attractive is your intermolecular forces lesson plan? Draw your class in with an activity that includes research, presentation, and demonstrations. Chemistry scholars work together to create claims about the each intermolecular...
Teach Engineering
Balancing Liquid on a Coin: How Intermolecular Forces Work
Let knowledge of chemistry flow like water. Future scientists conduct two different experiments to investigate the properties of water. They learn about surface tension and cohesion as they see how many drops of water they can place on a...
Chemistry Collective
Brownian Motion
Explore particle motion between solute and solvents. An interactive simulation allows learners to observe the motion of solute particles as they interact with the solvent particles. It provides an option for including Brownian dynamics...
Royal Society of Chemistry
The Treatment of Oil Spills—Microscale Chemistry
When oil spills happen, how is the oil cleaned up? Pupils of polymer science discover an amazing substance that turns oil into a solid during a microscale experiment. Individuals observe oil or paraffin before and after addition of the...
Royal Society of Chemistry
Investigating Temperature Changes on Evaporating Liquids—Microscale Chemistry
Is there more to evaporation than just less liquid? Show young scientists the energy transformation that occurs during a phase change through a series of simple experiments. Lab partners place drops of water, ethanol, and ethoxyethane on...
Concord Consortium
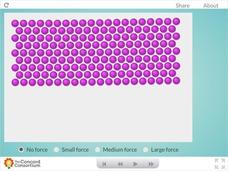
Metal Forces
Are you all bent out of shape, trying to find a great resource that illustrates the properties of metals? Show science scholars the unique world of metallic bonding with a hands-on activity. Users apply three levels of force to a sample...
Concord Consortium
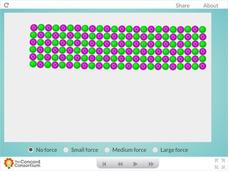
Ceramic Forces
Why are bricks more likely to break than bend? Young science scholars peer inside a ceramic block and examine the effects of downward force at the molecular level. Learners can apply three different levels of force before observing their...
Concord Consortium
Boiling Point of Polar and Non-Polar Substances
Go to extremes to illustrate boiling point! Junior chemists explore the effects of heating and cooling on polar and non-polar substances. The interactive allows users to raise and lower the temperature, set specific temperatures, and...
Concord Consortium
Boiling Point
Is it getting hot in here? Observe boiling from inside a beaker in an engaging interactive. Chemistry scholars heat and cool polar and non-polar solids and observe how molecules react to temperature changes. Your class' misconceptions...
Concord Consortium
Factors Affecting London Dispersion Attractions
How can non-polar molecules be attracted to one another? Introduce the phenomenon of London dispersion forces to young chemists through an entertaining interactive. Pupils choose from a variety of molecular shape combinations, then pull...
Concord Consortium
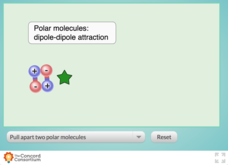
Polarity and Attractive Strength
Teaching intermolecular forces can be quite a stretch! Chemistry scholars experiment with the attractive strength between polar molecules using an interactive resource. Learners test molecules of low, medium, and high polarities by...
Concord Consortium
Oil and Water
If you don't get along with someone, it's said that the two of you are like oil and water. Why is this? Explore the phenomenon and explain the phrase in one resource! Science superstars first observe samples of oil and water together....
Concord Consortium
Comparing Dipole-Dipole to London Dispersion
Which intermolecular force is the strongest? Scholars test the relative strength of London dispersion forces, dipole-dipole interactions, and induced dipoles using a simulator. The interactive allows learners to pull on paired molecules...
Concord Consortium
Seeing Intermolecular Attractions
Ahh, the rules of attraction...intermolecular attraction! Introduce your chemistry crew to the other forces that influence the behavior of atoms and molecules alike with a simple interactive. Pupils push and pull polar and non-polar...
Concord Consortium
Intermolecular Attractions and States of Matter
Need a solid resource for teaching about states of matter? Science scholars go with the flow in a simple interactive that shows how intermolecular attractions determine a substance's phase. Pupils take control of the level of attraction...
Concord Consortium
Intermolecular Attractions and Boiling Point
Why do different substances have different boiling points? Through an interactive lesson, learners explore how intermolecular attractions affect boiling points. They interact with molecules through an animation and make conclusions about...
American Chemical Society
The Energy of Evaporation
Do all liquids evaporate at the same rate? Young scientists observe the evaporation rate of three different liquids. They measure the time, the temperature, and the change in energy. After comparing the chemical formulas, scholars...
National Institute of Open Schooling
Solid State
Crystal comes from a Greek word meaning ice. Activity eight in a series of 36 has class members analyzing amorphous and crystalline solids and further classifying them based on their forces. They then explore solids, learning about unit...
Science Geek
Intermolecular Forces of Attraction
Chemists love London (dispersion forces)! Presentation begins with an explanation of intermolecular forces including hydrogen bonding, dipole-dipole attraction, and London dispersion forces. It also covers polarity and the relative...
Curated OER
Review Set
The topics covered in these multiple choice questions are about atomic structure and bonding, state configurations, pressure and solution concentration, and energy graphs. This is a midterm review which could be used with the whole...
Curated OER
How Do Atoms Stick Together?
In this chemical bonding worksheet, students answer 76 questions about compounds, Lewis dot structures, intermolecular forces between atoms, electronegativity and bonding and types of bonds.
Curated OER
Solutions and Solubility Review
In this solutions and solubility review worksheet, students are given main ideas about intermolecular forces, concentrations of solutions, molar solutions, ionic equations, solubility rules, acids and bases and titrations. Students...