Curated and Reviewed by
Lesson Planet
This Comparing Dipole-Dipole to London Dispersion interactive also includes:
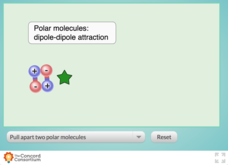
Which intermolecular force is the strongest? Scholars test the relative strength of London dispersion forces, dipole-dipole interactions, and induced dipoles using a simulator. The interactive allows learners to pull on paired molecules and observe how much force they need to apply to disrupt the attraction.
13 Views
8 Downloads
NGSS:
Adaptable
Additional Tags
Instructional Ideas
- Assign the interactive and ask the class to draw their own conclusions about the strength of intermolecular forces
- Extend the lesson by having pupils infer how each attractive force affects quantities like boiling point and melting point
Classroom Considerations
- Begin the activity by discussing how induced dipoles form between molecules
Pros
- Interactions between molecules are portrayed in a realistic manner
- Each molecule pair is labeled with the appropriate intermolecular force users observe
Cons
- None