SciShow
Exotic Chemistry: World's Oldest Water and The Rarest Element
This week's SciShow news brings you discoveries involving two of the most exotic substances on Earth - the world's rarest element and the world's oldest water. Two great tastes that taste great together? Stay tuned to find out.
SciShow
Exotic Chemistry: World's Oldest Water and The Rarest Element
This week's SciShow news brings you discoveries involving two of the most exotic substances on Earth - the world's rarest element and the world's oldest water. Two great tastes that taste great together? Stay tuned to find out.
Bozeman Science
Periodicity
In this video Paul Andersen explains why atoms in the periodic table show trends in ionization energy, atomic radii, electronegativity and charge. All of these trends are explained through Coulomb's Law. A brief description of Dmitri...
Bozeman Science
Coulomb's Law
In this video Paul Andersen explains how we can use Coulomb's law to predict the structure of atoms. These predictions can be verified through the use of Photoelectron Spectroscopy (PES). Electron's are help around the nucleus because of...
Bozeman Science
Electron Configuration
In this video Paul Andersen explains how to write out the electron configuration for atoms on the periodic table. More importantly he shows you why electrons arrange themselves in shells, subshells and orbitals by using Coulomb's law and...
Curated Video
What in the world is IONIZATION ENERGY?!?
Ionization energy is the amount of energy it takes to remove an electron. The stronger the nucleus can hold on to the electron, the more energy that will be needed to remove the electron. The trend for ionization energy is that it...
Curated Video
Charges in Atoms, Atomic Models, and Quantum Numbers
This video explains how the different particles in an atom have charges, how the different particles affect the overall charges of an atom, and how atomic models are created.
FuseSchool
Shielding
Learn the basics about shielding as a part of the atomic structure, within the overall properties of matter topic.
Professor Dave Explains
Covalent Bond Energy and Length
We've already learned about different types of chemical bonds, including covalent bonds. But now that we know about enthalpy, and orbitals, and some other concepts, let's revisit the covalent bond and dig a little deeper.
Professor Dave Explains
Practice Problem: Ionization Energy
When we learned about periodic trends, we learned about ionization energy. Just how much energy is required to remove an electron from an atom? What about a second electron, or a third? Let's compare a few different ionizations and see...
Professor Dave Explains
IIT/JEE Chemistry Practice #14: Ionization Energy
Practice REAL problems from actual past IIT/JEE exams with Professor Dave!
msvgo
Trends in Physical Properties of Group 13 Elements
It describes the properties of boron family compounds It discusses about atomic radii and ionic radii It explains the trends of ionisation enthalpy and electronegativity.
FuseSchool
How Does The Periodic Table Work
Learn the basics about the periodic table, and how the elements are organised within it in this video. The periodic table consists of 7 rows called Periods, going across, and 18 columns, called Groups going down. The elements in the...
Mazz Media
Stability and Chemical Bonds
The relationship between energy levels and strength of chemical bonds are discussed and demonstrated in this video. Students will learn that a chemical bond forms when electrons have lower energy and that less stable atoms tend to form...
Professor Dave Explains
The Periodic Table: Atomic Radius, Ionization Energy, and Electronegativity
Why is the periodic table arranged the way it is? There are specific reasons, you know. Because of the way we organize the elements, there are special patterns that emerge. And you know how Professor Dave feels about patterns. He likes...
Fuse School
Shielding
An atom's ability to lose an electron or attract an electron is not created equal! A thorough video lesson explains the concept of shielding and electron affinity. Learners understand the farther an electron is from the nucleus, the...
SciShow
Exotic Chemistry: World's Oldest Water and The Rarest Element
The oldest water, found buried in a mine, serves as a time capsule of the environment that existed 2.6 billion years ago. An interesting video explores science news from 2013—more specifically, the oldest water ever discovered and the...
TED-Ed
The Most Radioactive Places on Earth
Who receives that greatest amount of radioactive exposure? After visiting some of the most radioactive places on earth, including Chernobyl and Fukushima, viewers consider the exposure of radiation workers, astronauts, and cancer...
Berkeley University of California
Ionization Energy, Electron Affinity
What do dipoles say in passing? Have you got a moment? Videos begin with ionization energy, then move on to covalent bonds, polar-covalent bonds, and ionic bonds. After a discussion of dipole moments, a video quiz reviews the material....
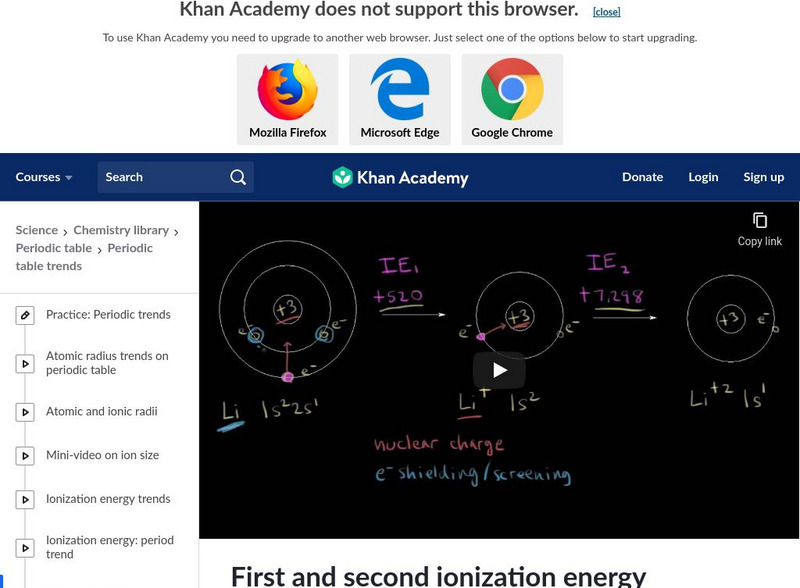
Khan Academy
Khan Academy: First and Second Ionization Energy
An explanation of the reasoning why there is such a large difference between first and second ionization energies. [7:34]
Khan Academy
Khan Academy: Electron Affinity
Electron affinity is defined. Electron affinities of various elements are compared. [11:39]
Khan Academy
Khan Academy: Chemistry: Other Periodic Table Trends
A video lecture discussing the trends on the periodic table dealing with ionization energy, electronegativity, metallic nature, and atomic radius size. The video shows how electronegativity increases to the top right of the periodic...
Khan Academy
Khan Academy: Chemistry: Periodic Table Trends: Ionization Energy
The energy to remove an electron, or ionization energy, is explained. [12:13]
Khan Academy
Khan Academy: Group Trends for Ionizations Energy
Explanation of the ionization energy required for different groups on the Periodic Table. [10:18]