Hi, what do you want to do?
Berkeley University of California
Solubility (NB)
Focus on the basics of solubility in chemistry with an explanatory video, which focuses on how to solve solubility problems and common ion effect calculations.
Crash Course
The Ideal Gas Law
Can you crush a soda can using only the air around you? Use an engaging video to teach about the relationship between pressure, volume, moles, and temperature of a given gas, all done with the Ideal Gas Law.
Berkeley University of California
Stoichiometry
Can you say stoichiometry five times quickly? Watch an informative video to learn the basics of stoichiometry, including how to pronounce it. Viewers also learn the relationship between the number of grams, number of moles, and...
Get Chemistry Help
Chemistry Lesson: Mole Calculations I
Do your pupils already have knowledge of moles and mass? If so, this is the next step for the chemistry classroom. The video segment, while short in length, is filled with demonstrations on how to perform mole calculations involving both...
Get Chemistry Help
Chemistry Lesson: The Mole (Avogadro's Number)
Avagodro's number (The Mole) ... you are not researching that cute little mole on your cheek! Avagodro's number refers to The Mole, a vital component to chemistry. This video segment will discuss the mole and further elaborate on...
BioEd Online
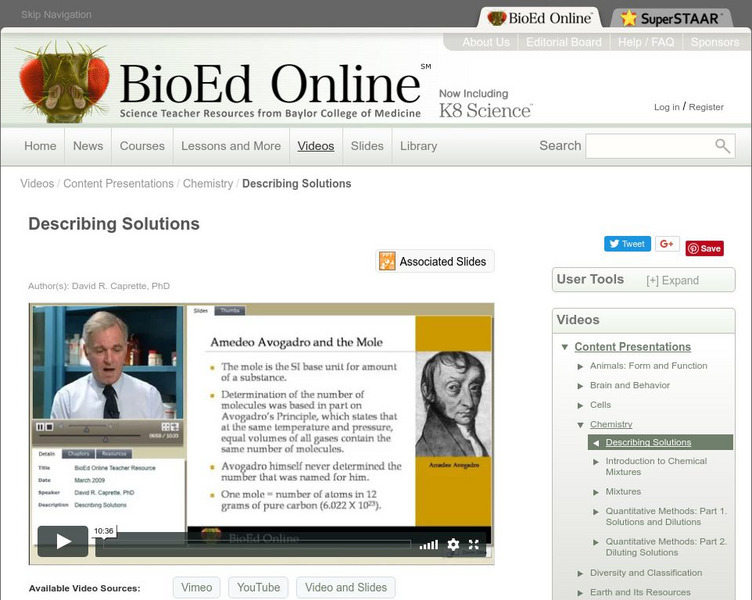
Bio Ed Online: Describing Solutions
Most scientists use a common language of measurement, called the International System of Units, that is recognized in labs all over the world. In thie following video David Caprette, PhD, explains this language and its well-established...
BioEd Online
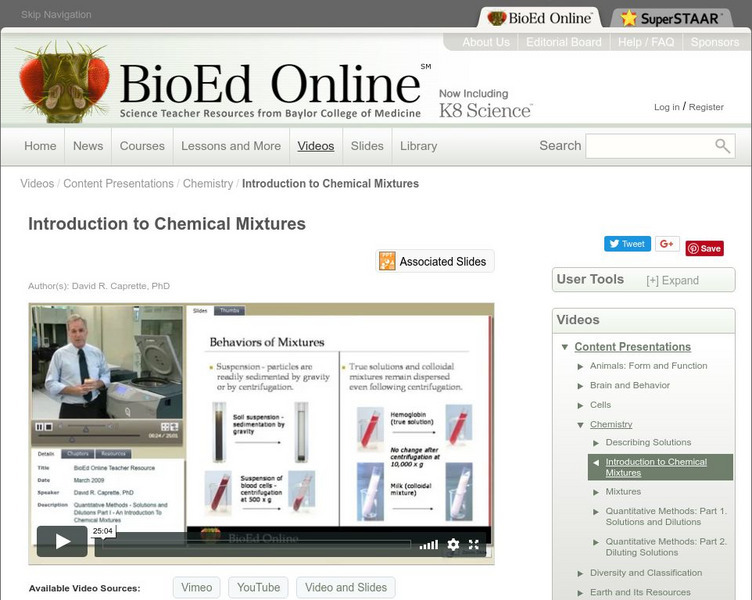
Bio Ed Online: Introduction to Chemical Mixtures
A biologist must be able to work with and understand a variety of mixtures. In the following video David Caprette, PhD, describes types of mixtures, and their properties, that might be encountered in a biological laboratory, and...
Sophia Learning
Sophia: Converting From Moles to Volume
This lesson will provide examples of converting from moles to volume of a gas.
Sophia Learning
Sophia: Converting From Volume to Moles
This lesson will provide examples of converting from volume of a gas to moles of a gas.
Khan Academy
Khan Academy: The Mole and Avogadro's Number
Video tutorial presents an introduction to the concept of a mole as a number. [9:43]
Khan Academy
Khan Academy: Ideal Gas Equation Example 1
Figure out the number of moles of gas we have using the ideal gas equation: PV=nRT. [10:08]
Tyler DeWitt, PhD
Tyler De Witt: Converting Between Moles, Atoms, and Molecules
How many atoms in 5.5 moles? How many moles is 4.6 x 10^24 sulfur atoms? In this video Tyler DeWitt helps you solve problems like these--converting back and forth between moles and the number of atoms or molecules. DeWitt will use both a...
Tyler DeWitt, PhD
Tyler De Witt: Converting Between Moles, Atoms and Molecules Part 2
In this video viewers will do more practice problems, converting between moles, atoms, and molecules. This is the second part of the video. [6:58]
Khan Academy
Khan Academy: Chemistry: Ideal Gas Equation Example 1
A video lecture applying the ideal gas equation to solve for the number of moles contained in a balloon. [10:08]
Sophia Learning
Sophia: Ideal Gas Law Equation: Lesson 2
This lesson will explain the variables in the ideal gas law equation. It is 2 of 2 in the series titled "Ideal Gas Law Equation."
Sophia Learning
Sophia: Ideal Gas Law Equation Examples: Lesson 2
This lesson will present several example math problems utilizing the ideal gas law. It is 2 of 2 in the series titled "Ideal Gas Law Equation Examples."
Sophia Learning
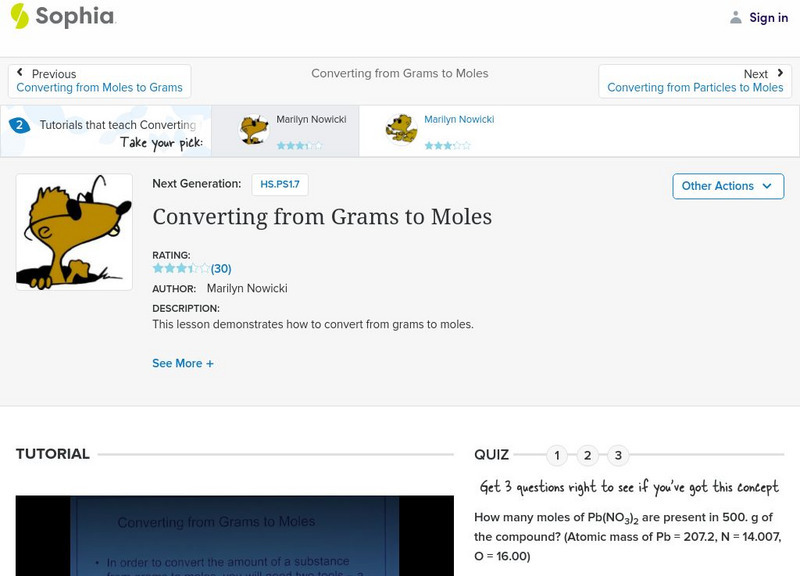
Sophia: Converting From Grams to Moles: Lesson 1
This lesson demonstrates how to convert from grams to moles. It is 1 of 2 in the series titled "Converting from Grams to Moles."
Other
Mad Penguin Films: A Mole Is a Unit
Watch this short video to get an idea of just how big Avogadro's number is. The examples are certainly clear, and the little tune is catchy. [3:49]
Tyler DeWitt, PhD
Science With Tyler De Witt: Counting Atoms: Intro to Moles Part 2
Learn about moles to figure out how many atoms you have in something using the periodic table, molar mass, and Avogadro's number. [10:08]
Tyler DeWitt, PhD
Science With Tyler De Witt: Introduction to Moles
A tutorial which explains what moles are and why they are important. Learn how to abbreviate the mole number (Avogadro's number) using scientific notation, and how giant this number is. [10:50]
Khan Academy
Khan Academy: Thermodynamics: Thermodynamics (Part 4)
An introduction to the concept of a mole and its role in the field of thermodynamics. Using a periodic table, one learns how to calculate the number of moles a quantity of an element has, based on its atomic mass. Goes on to explain how...
Khan Academy
Khan Academy: Molarity, Molality, Osmolarity, Osmolality, Tonicity Differences
See how each of these terms (molarity, molality, osmolarity, osmolality, tonicity) tells us something different about a solution in the Khan Academy video. [4:03]
Khan Academy
Khan Academy: 2015 Ap Chemistry Free Response 2 a (Part 1 of 2)
Review for the AP Chemistry exam with this video showing a worked free response problem. This problem is part 1 of 2 showing how to calculate how many moles of ethene is produced from dehydration reaction with ethanol.
Khan Academy
Khan Academy: Thermodynamics Part 4: Moles and the Ideal Gas Law
Sal explains the concept of a mole. Then he derives the molar version of the ideal gas law PV=nRT, where the gas constant R=831 J/molK. [10:14]