Hi, what do you want to do?
Brightstorm
Brightstorm: Science: Percent Yield
Video tutorial explains percent yield for students and provides information on how to calculate and solve chemical equations. [5:16]
Khan Academy
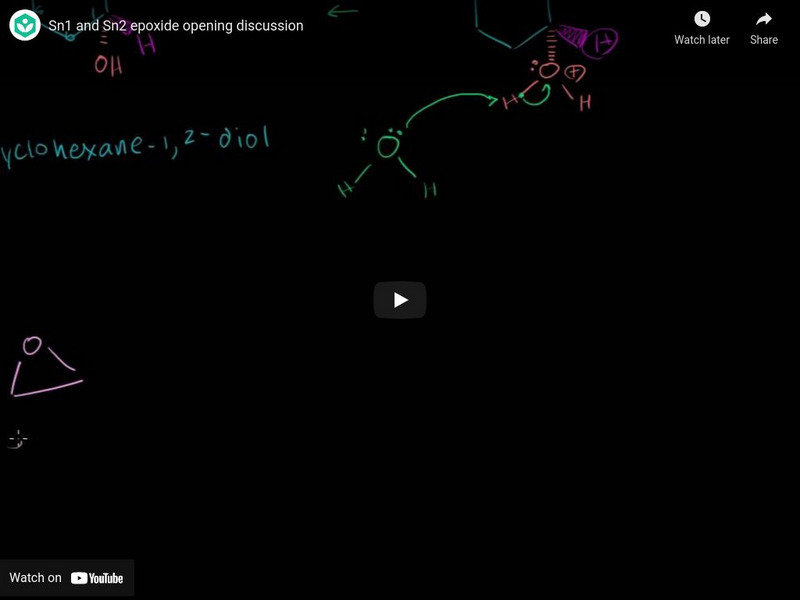
Khan Academy: Sn1 and Sn2 Epoxide Opening Discussion
Video lecture presents Sn1 and Sn2 epoxide opening discussion. [7:59]
Crash Course
Crash Course Kids 23.2: (Lego) Block Party
In this episode of Crash Course Kids, the host chats about chemical reactions and the conservation of matter. [3:52]
Khan Academy
Khan Academy: Sn1/sn2/e1/e2: Comparing E2 E1 Sn2 Sn1 Reactions
Looks at what type of reaction might occur if we have Bromocyclopentane dissolved in Dimethylformamide (DMF), with Methoxide ion in the solution. It could be an E2, E1, SN2, or SN1 reaction, and we look for clues to see which type is...
Khan Academy
Khan Academy: Free Radical Reaction: Free Radical Reactions
Looks at what would happen in a reaction if free radicals are present. [13:45]
Khan Academy
Khan Academy: Sn1/sn2/e1/e2: E2 E1 Sn2 Sn1 Reactions Example 3
Video lecture explores E2 E1 Sn2 Sn1 Reactions. Example 3. [13:18]
Khan Academy
Khan Academy: Ring Opening Reactions of Epoxides: Acid Catalyzed
Video lecture explores ring-opening reactions of epoxides when everything is acid-catalyzed. [8:41]
Khan Academy
Khan Academy: Synthesis of Alcohols: Preparation of Alcohols Using Li Al H4
Khan Academy explores the use of lithium aluminum hydride to prepare alcohols. [11:17]
Khan Academy
Khan Academy: Ring Opening Reactions of Epoxides: Strong Nucleophiles
Observe the formulae for chemical reactions and bonding utilizing strong nucleophiles. [14:07]
Khan Academy
Khan Academy: Reactions of Alcohols: Biological Redox Reactions
This video looks at the biological redox reactions of alcohols and phenols. [9:46]
Khan Academy
Khan Academy: Sn1/sn2/e1/e2: E2 E1 Sn2 Sn1 Reactions Example 2
A second example that looks at what type of reaction we might have - E2, E1, SN2, or SN1 - given certain ingredients. [3:06]
Bozeman Science
Bozeman Science: Conservation of Charge in Reactions
In the following video Paul Andersen explains how the charge is conserved in nuclear reactions. When elementary particles are created or destroyed in a reaction the net change in charge will remain constant. Alpha, beta -, and beta+...
The Kid Should See This
Tksst: Beautiful Chemical Reactions
See how beautiful chemical reactions can be, and observe metal displacement, precipitation, crystallization, and more. [6:30]
Bozeman Science
Bozeman Science: Chemical Reactions
A video exploring how bonds are broken and formed during chemical reactions. Learn about the collision theory, changes in state, and changes in molecules effect chemical reactions. [7:28]
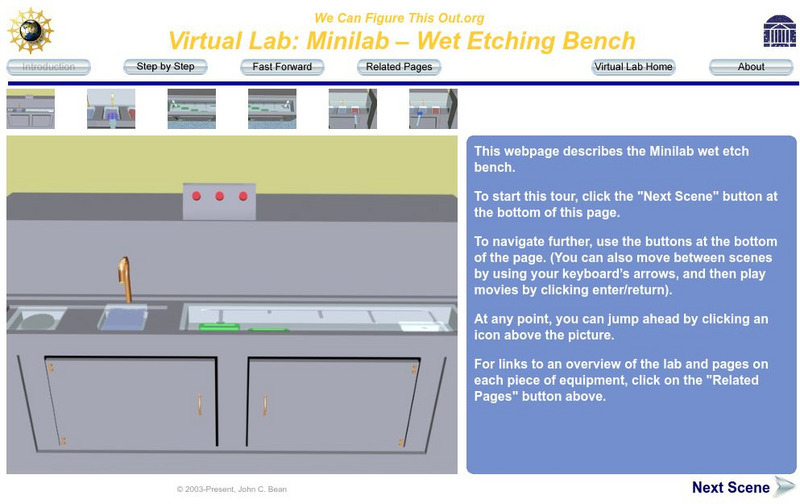
University of Virginia
Uva Virtual Lab: Wet Etching Bench
A process of removing material using liquid chemicals is featured in this video series. Understand what wet etching is.
Khan Academy
Khan Academy: Balancing Chemical Equations
A video [5:03] showing how to balance a chemical reaction by making sure you have the same number of atoms of each element on both sides.
Khan Academy
Khan Academy: Balancing More Complex Chemical Equations
A video [4:37] showing how balance more complicated reactions by of balancing the combustion reaction of ethylene.
Khan Academy
Khan Academy: Balancing Chemical Equation With Substitution
A video [4:11] showing how to use the substitution method to balance a reaction where a reactant and product have a polyatomic ion in common.
Khan Academy
Khan Academy: Chemical Reactions Introduction
Reactants and products in reversible and irreversible chemical reactions. [9:12]
Khan Academy
Khan Academy: Redox Titration
The resource from Khan Academy provides practice questions for the AP Chemistry course. A redox titration example: titrating an Fe(II) solution with potassium permanganate.
Khan Academy
Khan Academy: Titration Introduction
This video is an introduction to acid-base titrations using example of titrating 20.0 mL of HCl of unknown concentration with 0.100 M NaOH. Covers indicators, endpoint, equivalence point, and calculating the unknown concentration.
Next Vista for Learning
Next Vista for Learning: Chemistry Chemical Reactions
A video demonstrating how to do an experiment showing a chemical reaction. [1:33]
Sophia Learning
Sophia: Reaction Mechanism
This lesson will define reaction mechanism, and introduce the concept that reactions do not all occur in one step, and provide an example of a multistep reaction, along with how this is demonstrated on a reaction progress diagram....