Professor Dave Explains
Anti-Markovnikov Hydrohalogenation
Looking at anti-markovnikov hydrohalogenation as an example of a radical reaction.
Mazz Media
Electron Energy Levels and Valency
In this video students will learn that an electron energy level or energy shell is the orbit followed by electrons around an atom’s nucleus and that the number of electrons within these shells or energy levels balances out the positive...
FuseSchool
Polymerisation of Ethene
Learn the basics about the polymerisation of ethene as a part of organic chemistry.
Curated Video
Atoms and their Interactions - The Chemistry of Life
In this section, I talk about elements, atoms and how they interact. I deal with two types of bonds: Covalent Bonds and Ionic Bonds. I also talk about pH, chemical reactions and metabolism.
Professor Dave Explains
Electrophilic Aromatic Substitution
Introducing electrophilic aromatic substitution.
msvgo
Covalent Compounds-Lewis structures
It explains the Kossel-Lewis approach to chemical bonding, octet rule, covalent bond and classify its different types, Lewis dot structures of covalent compounds.
Professor Dave Explains
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Ionic Bond, Covalent Bond, James Bond, so many bonds! What dictates which kind of bond will form? Electronegativity values, of course. Let's go through each type and what they're all about.
FuseSchool
What Are Intermolecular Forces
Learn what intermolecular forces are, the three most common types and the differences between them. An intermolecular force is simply an attractive force between neighbouring molecules. There are three common types of intermolecular...
Visual Learning Systems
Understanding Covalent Bonds
This video explains the concept of covalent bonds and how they are formed between atoms. It uses examples of hydrogen and chlorine bonding, as well as the formation of water through covalent bonding between hydrogen and oxygen atoms....
FuseSchool
What Are Covalent Bonds
Learn the basics about covalent bonds, when learning about properties of matter. When similar atoms react, like non-metals combining with other non-metals, they share electrons. This is covalent bonding. Non-metals have shells of...
TED-Ed
How Polarity Makes Water Behave Strangely
Water is common? Not really! Learn how the polarity of the water molecule gives it tremendous properties that make is quite unique in the universe. Learners will understand surface tension, adhesion, and cohesion, as well as why these...
Curated OER
Ionic and Covalent Bonding Animation
This nifty little presentation uses computer animation to illuminate how ionic molecular bonds and covalent bonds are formed. This would be a terrific addition to your PowerPoint or Smart Board lesson on molecular bonding.
Massachusetts Institute of Technology
Mit: Blossoms: Plastics and Covalent Chemical Bonds
Interactive video lesson discusses the distribution of electrons in a carbon atom, and then follows with a chemical investigation of plastics.
PBS
Pbs Learning Media: What Holds a Molecule Together?
This video/animation illustrates that a molecule as a small group of atoms stuck or bonded together with electrons. Dr. Chris Muhlstein introduces the idea of these three primary types of bonds: ionic, covalent and metallic; animations...
Khan Academy
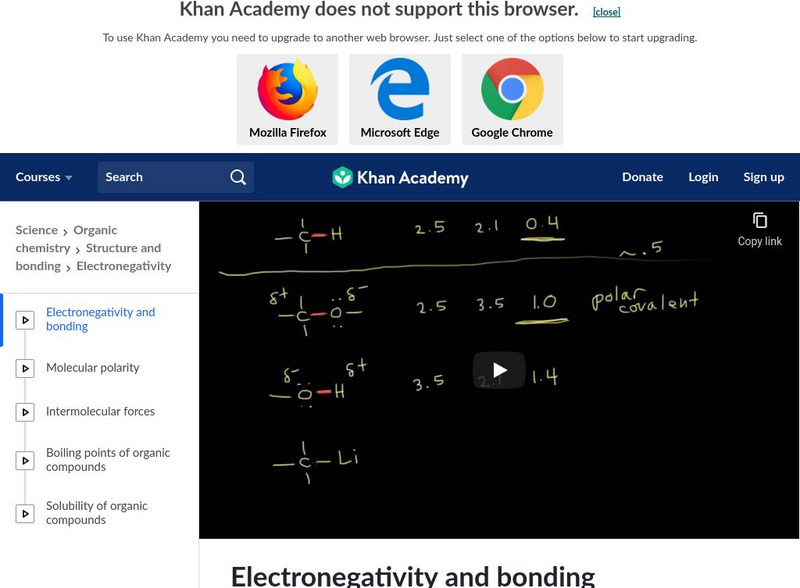
Khan Academy: Structure and Bonding: Electronegativity and Bonding
Learn how to classify bonds as covalent, polar covalent or ionic by using the differences in electronegativity in this video. Video explains the Pauling scale to find the electronegativity differences in bonding. [11:38]
Khan Academy
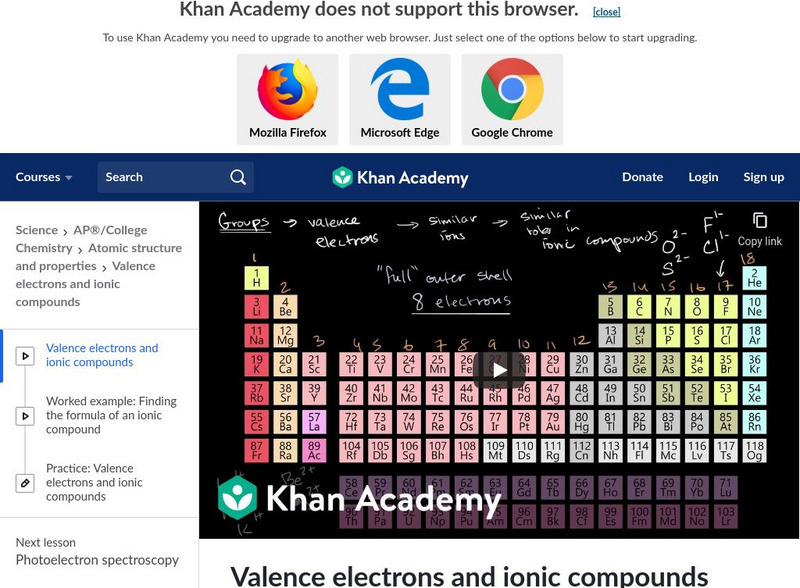
Khan Academy: Valence Electrons and Bonding
An explanation of how the amount of electrons in the outermost shell of an atom designates the reactivity to other elements. [10:57]
Khan Academy
Khan Academy: Electronegativity
Electronegativity is defined and compared to electron affinity. Trends based on groups and periods are also explored. [9:54]
Khan Academy
Khan Academy: Chemistry: Other Periodic Table Trends
A video lecture discussing the trends on the periodic table dealing with ionization energy, electronegativity, metallic nature, and atomic radius size. The video shows how electronegativity increases to the top right of the periodic...
National Science Foundation
National Science Foundation: Chemistry of Water
Video that explores the properties of the water molecule as a "universal" solvent. Content is explained in plain, easy-to-understand language with simple analogies for young chemistry students. [4:46]
Khan Academy
Khan Academy: Chemistry: Ionic, Covalent, and Metallic Bonds
A video lecture investigating the basics of chemical bonding. The video shows how bonds are formed by giving up or taking an electron, sharing electrons, and between metals. Examples of each type (ionic, metallic, and covalent) are...
Other
Capella University: Periodic Table of the Elements: Ionic and Covalent Bonds
A narrated introduction to ionic and covalent bonds through the platform of an animated periodic table of elements. [4:40]