Bozeman Science
Environmental Systems
In this video Paul Andersen explains how matter and energy are conserved within the Earth's system. Matter is a closed system and Energy is open to the surroundings. In natural systems steady state is maintained through feedback loops...
Khan Academy
Khan Academy: Enthalpy
An explanation of how enthalpy in a constant pressure system can be considered heat content. [15:07]
Khan Academy
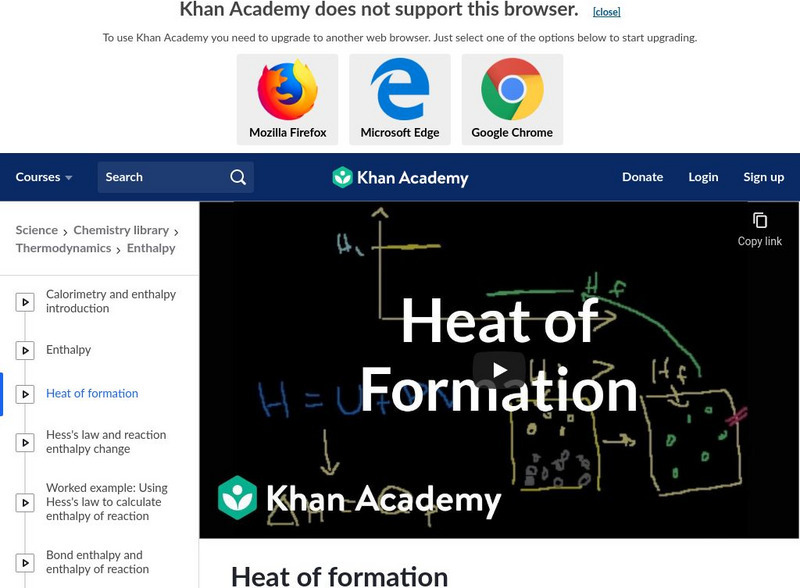
Khan Academy: Heat of Formation
An explanation to show how heats of formation are calculated. [12:35]
Khan Academy
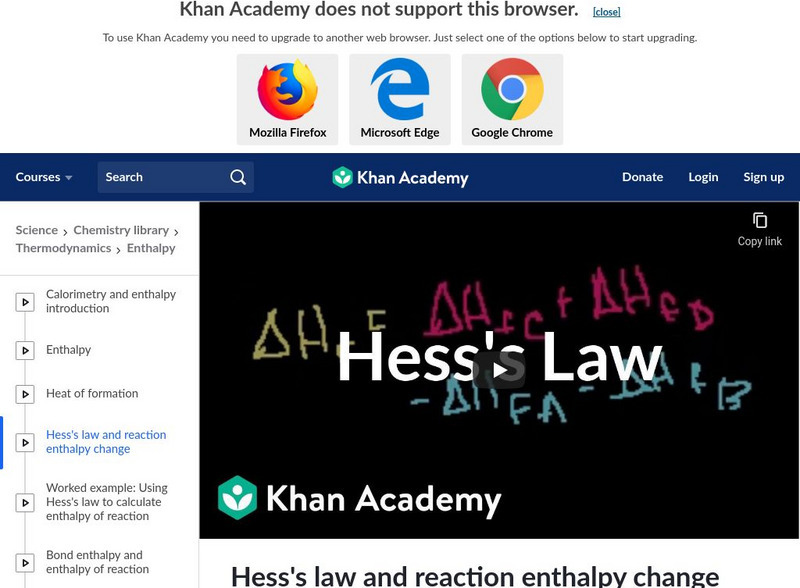
Khan Academy: Hess's Law and Reaction Enthalpy Change
Using Hess's Law and standard heats of formation to determine the enthalpy change for reactions. [15:40]
Khan Academy
Khan Academy: Hess's Law Example
An example using Hess's Law to calculate enthalpy change. [12:08]
Khan Academy
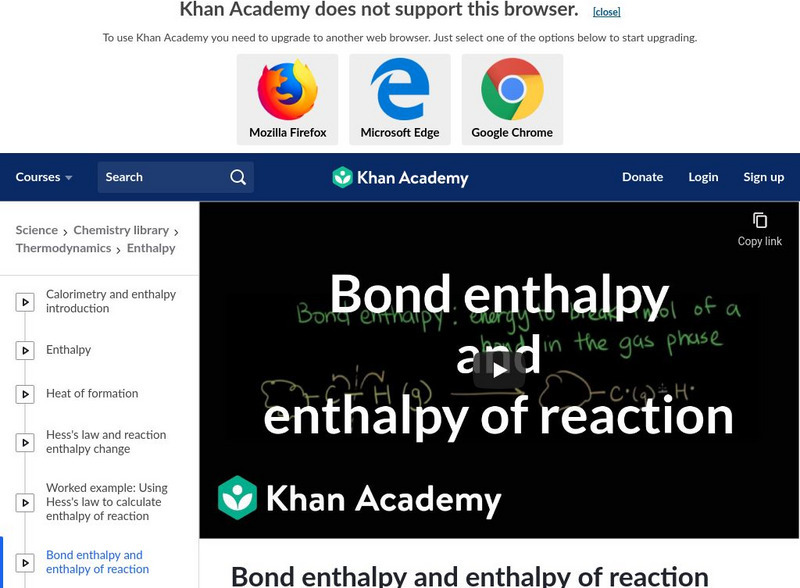
Khan Academy: Bond Enthalpy and Enthalpy of Reaction
An explanation of using bond enthalpy to calculate enthalpy of reaction. [11:47]
Khan Academy
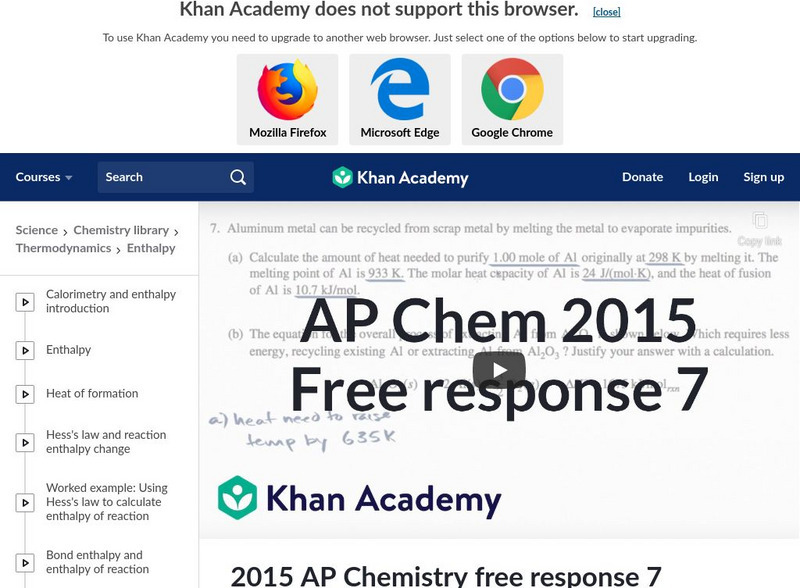
Khan Academy: 2015 Ap Chemistry Free Response 7
Calculating the necessary energy to recycle aluminum from aluminum oxide which was a question on the 2015 AP Chemistry exam. [6:16]
Khan Academy
Khan Academy: Second Law of Thermodynamics
An explanation of the Second Law of Thermodynamics. [10:22]
Khan Academy
Khan Academy: Work Done by Isothermic Process
Calculating the amount of work done to compare the heat added to a system and the isothermic process. [19:03]
Khan Academy
Khan Academy: Carnot Cycle and Carnot Engine
An introductory explanation of the Carnot Cycle and the Carnot Heat Engine. [20:52]
Khan Academy
Khan Academy: Proof: Volume Ratios in a Carnot Cycle
An explanation to prove the volume ratios in a Carnot cycle. [17:08]
Khan Academy
Khan Academy: Proof: S (Or Entropy) Is a Valid State Variable
An explanation to prove that entropy, labeled as S, is a valid state variable. [15:38]
Khan Academy
Khan Academy: More on Entropy
An explanation showing the differences between microstates and macrostates emphasizing that entropy is defined for a system and not different microstates. [8:56]
Khan Academy
Khan Academy: Maxwell's Demon
Explanation of a concept that seems to defy the Second Law of Thermodynamics. [13:27]
Khan Academy
Khan Academy: Gibbs Free Energy Example
Calculating change in Gibb's free energy to identify whether or not a reaction is spontaneous. [9:56]
Khan Academy
Khan Academy: More Rigorous Gibbs Free Energy / Spontaneity Relationship
Extended explanation of the effect of a negative charge on Gibbs Free Energy. [13:56]
Khan Academy
Khan Academy: A Look Ar a Seductive but Wrong Gibbs/spontaneity Proof
An explanation why many textbooks are incorrect in their details about the "proof" of the relation between Gibbs free energy state and spontaneity. [6:41]
Khan Academy
Khan Academy: Changes in Free Energy and the Reaction Quotient
An explanation of Gibbs Free Energy and the reaction quotient under conditions that are not standard. [15:47]
Khan Academy
Khan Academy: Biology: Energy and Enzymes: Second Law of Thermodynamics
Learn that heat can not be spontaneously transferred from cold to hot based on the Second Law of Thermodynamics in this video. [10:22]
Khan Academy
Khan Academy: Energy and Enzymes: Second Law of Thermodynamics and Entropy
Learn that the entropy of the universe is constantly increasing according to the second law of thermodynamics in this video. [8:32]
Khan Academy
Khan Academy: Entropy: Embrace the Chaos
An explanation regarding the disorder and organized states that occur as energy is transferred and conserved within systems and reactions. [12:32]
Khan Academy
Khan Academy: Thermodynamic Entropy Definition Clarification
Using a reversible system to clarify the definition of entropy in thermodynamics. [15:38]
Khan Academy
Khan Academy: Reconciling Thermodynamic and State Definitions of Entropy
Explanation of how entropy relates to the number of states a system can take on. [28:26]
Khan Academy
Khan Academy: Entropy Intuition
An introduction to the Second Law of Thermodynamics focusing on terms of heat and microstates. [19:15]