Hi, what do you want to do?
Curated OER
Atomic Emission Spectra and the Bohr Model of the Atom
Students investigate the wavelengths of spectra lines for common atoms. For this atomic emission spectra lesson plan, students use a transformer and diffraction grating to observe the emission spectra of various atoms. They find the...
Curated OER
Typical Numeric Questions for Physics I - Atomic Spectra
Seven practice problems are presented to physics pros in this assignment. Given the wavelengths, they perform computations for emission spectra. This brief worksheet makes an appropriate pop quiz.
Curated OER
Atomic Absorption Determination of Zinc and Copper in a Multivitamin
Advanced lab apprentices prepare zinc and copper solutions to which they will compare the same minerals from a multivitamin. Using absorption spectroscopy, they analyze the contents of the multivitamin for concentration. This lab can be...
Curated OER
Discovery 4-1 Atomic Emission Spectra
In this emission spectra worksheet, learners use a spectroscope to observe three types of spectra. These include continuous spectrum, emission spectrum and absorption spectrum.
Curated OER
Organic Chemistry Problem Set Exam 1
Though there are technically only 13 questions on this exam, they take up six pages and make a thorough assessment of organic chemistry principles. There are plenty of diagrams to label or complete. Emission spectra are displayed for...
Curated OER
2009 U.S. National Chemistry Olympiad National Exam - Part I
The 2009 version of the first part of a national chemistry competition is posted for your use with olympiad hopefuls. Test takers deal with 60 multiple choice questions covering an entire year of chemistry curriculum. Use this to...
Curated OER
Atomic Structure
Students are introduced to moles and Avogadro's number, Bohr's model, Atomic emission spectra and quantum numbers. They also comprehend the current model of atoms and how electrons are described by quantum numbers.
Curated OER
Energy
Wow! Colorful and simple, these 160 slides introduce the various forms of energy, along with a relevant image. Some of the images are animations, which help beginning physical scientists to visualize the flow of electrons or energy! This...
Curated OER
AP Chemistry Atomic Structure-7 Worksheet
In this atomic structure worksheet, students solve twelve problems related to wavelength, frequency, electron transitions, emission spectra and quantum number.
Curated OER
Modern Physics, Old QT
In this physics worksheet, students develop an understanding of the atomic model and how atoms relate to one another through answering the seven questions.
Curated OER
Worksheet 4-1 Atomic Spectra
In this atomic spectra worksheet, students answer eighteen questions about wavelengths of light, the emission spectrum, energy of photons, the frequency of electromagnetic radiation and electrons in the excited state.
Curated OER
Flame Test
Students conduct a flame test on different substances. In this chemistry lesson, students predict the element present based on the color emitted during the flame test. They explain how different elements produce different colors.
Curated OER
Energy Dispersive Spectroscopy
Students calculate the values of electron binding energies. In this physics lesson, students solve for different wavelength characteristics of X-rays. They present their findings to the class.
Curated OER
Automated Vehicle Programming Design
Students design a program to make an automated vehicle perform a specific task. In this robotics lesson, students play the role of scientists competing to win a factory's contract. They present their algorithm to class.
Curated OER
Quantum Physics
Students discuss the mass-energy relationship based on Einstein's work. They calculate the energy released in various scenerios and sketch diagrams for the Lyman, Balmer and Pfund Series. In groups, they discuss the role of photons and...
CK-12 Foundation
Ck 12: Atomic Emission Spectra
[Free Registration/Login may be required to access all resource tools.] In this lesson, students learn about atomic emission spectra. Includes a simulation for exploring the Blackbody Spectrum.
CK-12 Foundation
Ck 12: Plix: Atomic Emission Lamps: Atomic Emission Spectra
[Free Registration/Login Required] In this interactive you will drag each gas bulb into the red circle to excite them and see their emission spectrum. You will need a sign-in to access this media, but it will be worth your time!
CK-12 Foundation
Ck 12: Light
[Free Registration/Login may be required to access all resource tools.] In the following tutorial students will describe the relationships between speed, wavelength, and frequency of light. They will understand the photoelectric effect...
Khan Academy
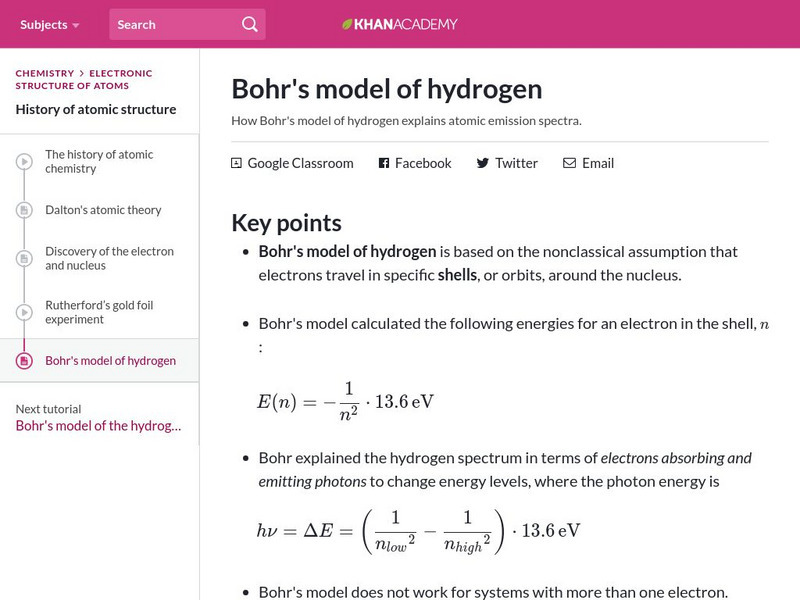
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
Friesian School
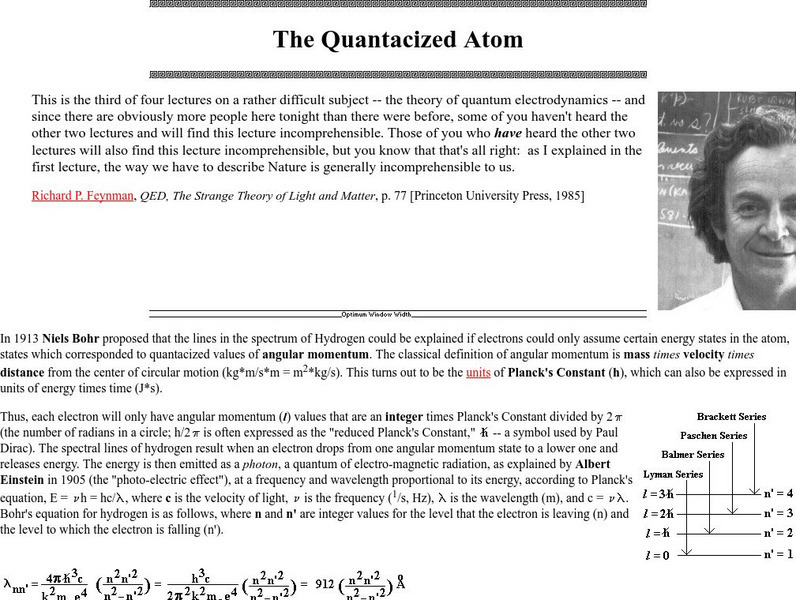
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
University of Colorado
University of Colorado: Physics 2000: Quantum Atom
Several pages with an interesting discussion of the visible light spectrum and atomic absorption and emission line spectrum. Features excellent graphics, thorough and understandable discussion, and many interactive Java applets.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
CK-12 Foundation
Ck 12: Chemistry Simulation: Neon Lights
[Free Registration/Login Required] Neon lights are a type of discharge tube. Observe how electrons create colored light in a hydrogen gas discharge tube. Can you figure out why hydrogen's emission spectrum contains more than one color of...