Hi, what do you want to do?
Curated OER
Worksheet 4-1 Atomic Spectra
In this atomic spectra worksheet, students answer eighteen questions about wavelengths of light, the emission spectrum, energy of photons, the frequency of electromagnetic radiation and electrons in the excited state.
Curated OER
Atoms, Molecules, and Chemical Bonds
In this atoms worksheet, students review the parts of an atom, Bohr diagram, atomic number, mass number, and covalent bonds. This worksheet has 5 drawings and 26 fill in the blank questions.
Curated OER
Using several learning modalities to teach about the Periodic Table.
Students identify how to relate the position of an element in the periodic table to its atomic number and atomic mass. They identify how to use the periodic table to identify metals, semimetals, nonmetals, and halogens, and also,...
Curated OER
Review for Chemistry
In this review for chemistry worksheet, learners decide if given statements are true or false. Students relate information learned about introductory knowledge gained in chemistry to accurately answer the given questions.
Curated OER
If My Configurations are Correct
Students write the electron configuration of elements in the ground state. In this chemistry lesson, students draw how subatomic particles are arranged in the atom. They construct Lewis dot diagrams of valence electrons.
Curated OER
Electrical Conduction in Semiconductors
In this electronics activity, students explore the properties of semiconductors to complete 17 short answer and problem solving questions.
Curated OER
Physical and Chemical Changes
Eighth graders view a PowerPoint presentation that assist them in distinguishing between physical and chemical changes. They compare their observations of demonstrations to a list of clues recognizing types of changes.
Curated OER
Static Cling
Students work together to discover the concept of static electricity. They participate in an experiment in which they test different objects charge. They make observations and record them for later use.
Curated OER
Quantum Physics
Students discuss the mass-energy relationship based on Einstein's work. They calculate the energy released in various scenerios and sketch diagrams for the Lyman, Balmer and Pfund Series. In groups, they discuss the role of photons and...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
CK-12 Foundation
Ck 12: Structure of the Atom
[Free Registration/Login may be required to access all resource tools.] Students learn about the important discoveries of subatomic particles, and how they led to our current understanding of the atom.
Sophia Learning
Sophia: Models of the Atom: Lesson 3
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 3 of 3 in the series titled "Models of the Atom."
Other
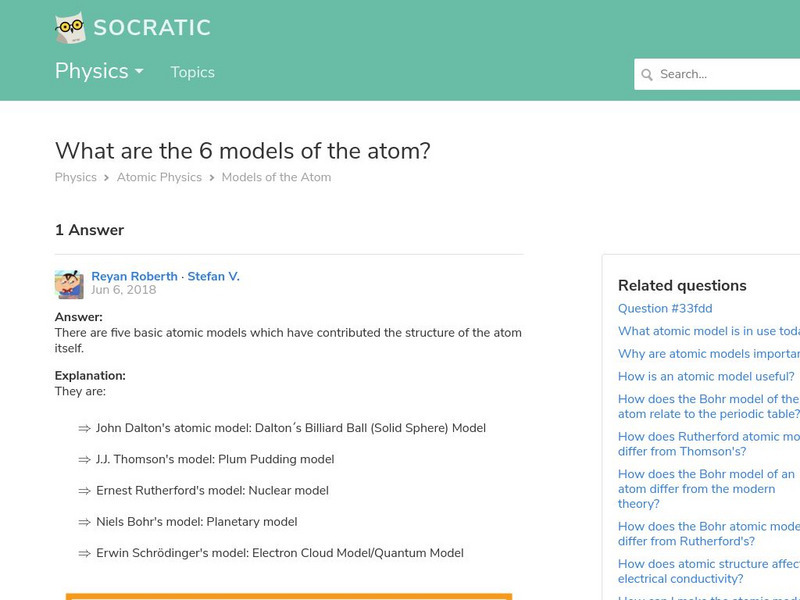
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
Science Education Resource Center at Carleton College
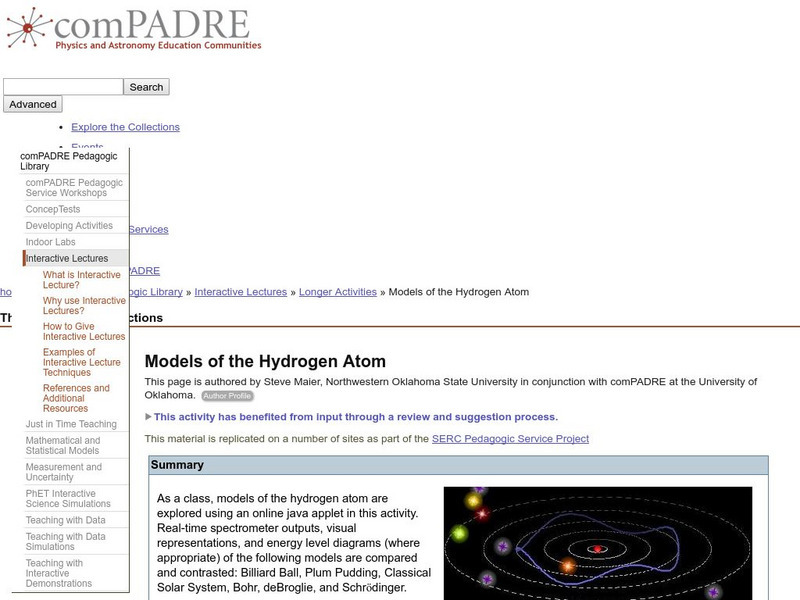
Serc: Models of the Hydrogen Atom
In this activity, students will explore several different models of the hydrogen atom and compare and contrast them using an online java applet.
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
Science Struck
Science Struck: The Structure of an Atom: A Labeled Diagram
Looks at the scientists who developed the model of the atom by building on previous scientists' discoveries about its structure.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Models of the Hydrogen Atom
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
Other
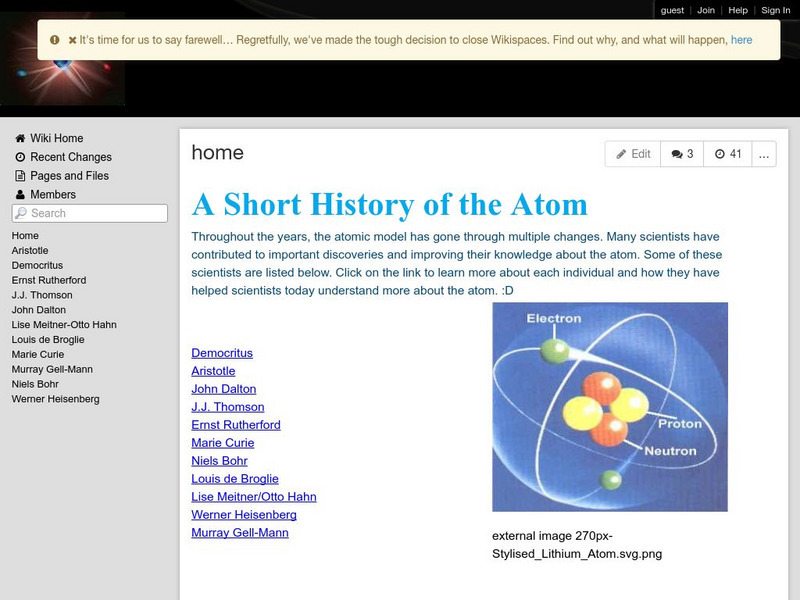
A Short History of the Atom
A classroom wiki where students present profiles of scientists who developed models of the atom or who contributed to the understanding of atomic theory. Covers Democritus, Aristotle, John Dalton, J.J. Thomson, Ernest Rutherford, Marie...
Sophia Learning
Sophia: Models of the Atom: Lesson 2
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 2 of 3 in the series titled "Models of the Atom."