Hi, what do you want to do?
University of Calgary
Univeristy of Calgary: Cycloalkanes
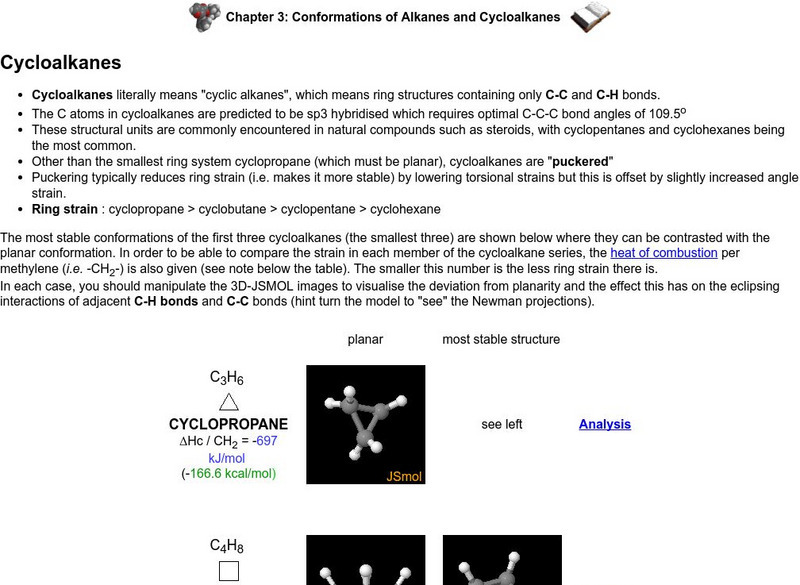
A brief description of cycloalkanes. Properties, chemical make-up, and structure provided. Brief introduction to nomenclature of cycloalkanes and generic formula. Interactive piece requires x-pdb plug-in.
Simon Fraser University
Chem1 Virtual Textbook: Chemical Composition
Chemical composition is a section of a larger overview on Chemistry, covering a variety of aspects. This section focuses on elements, atoms, compounds, and structure. Examples, formulas, and pictures are provided.
CK-12 Foundation
Ck 12: Ionic Compounds
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will name ionic compounds containing main group or transition metals, covalent compounds, acids, and bases, using...
CK-12 Foundation
Ck 12: Molecular Compounds
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will name ionic compounds containing main group or transition metals, covalent compounds, acids, and bases, using...