Bozeman Science
A Beginner's Guide to Balancing Equations
Mr. Andersen explains the basics of balancing chemical equations. A visual guide shows you how to change coefficients to balance the atoms in reactants and products.
Brian McLogan
Properties of logarithms for condensing an expression
👉 Learn how to condense logarithmic expressions. A logarithmic expression is an expression having logarithms in it. To condense logarithmic expressions means to use the logarithm laws to reduce logarithm expressions from the expanded...
Curated Video
Pure Substances vs. Mixtures: Understanding the Properties and Differences
The video discusses the concept of pure substances in chemistry and highlights the differences between pure substances and mixtures. The presenter explains that pure substances are defined as a single element or compound that is not...
Bozeman Science
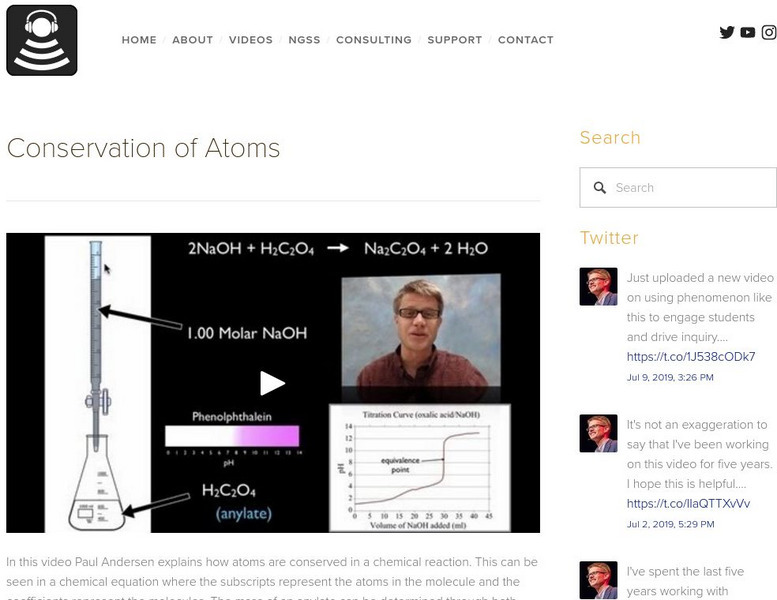
Conservation of Atoms
In this video Paul Andersen explains how atoms are conserved in a chemical reaction. This can be seen in a chemical equation where the subscripts represent the atoms in the molecule and the coefficients represent the molecules. The mass...
Sophia Learning
Sophia: Chemical Equation: Balancing: Lesson 2
This lesson will use examples to demonstrate how to balance a chemical equation using coefficients and leaving formula subscripts unaltered. It is 2 of 2 in the series titled "Chemical Equation: Balancing."
Bozeman Science
Bozeman Science: Ap Chemistry: Conservation of Atoms
Paul Andersen explains how atoms are conserved in a chemical reaction. This can be seen in a chemical equation where the subscripts represent the atoms in the molecule and the coefficients represent the molecules. The mass of an anylate...