University of Colorado
University of Colorado: Ph Et Interactive Simulations: Conductivity
Experiment with conductivity in metals, plastics and photoconductors. See why metals conduct and plastics don't, and why some materials conduct only when you shine a flashlight on them. Java required.
CK-12 Foundation
Ck 12: Metallic Bonds
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial, students will begin to describe the electron-sea model of metallic bonding. They will explain how metallic bonding is responsible...
Texas Education Agency
Texas Gateway: Chemical Bonding: Metallic Bonds
Given scenarios or diagrams, students will describe the nature of metallic bonding and explain properties such as thermal and electrical conductivity, malleability, and ductility of metals.
Chem4kids
Chem4 Kids: Metals in the Periodic Table
This site provides a general overview of the metals in the periodic table. Content explores the different types of metals, and how you can identify a metal.
Colorado State University
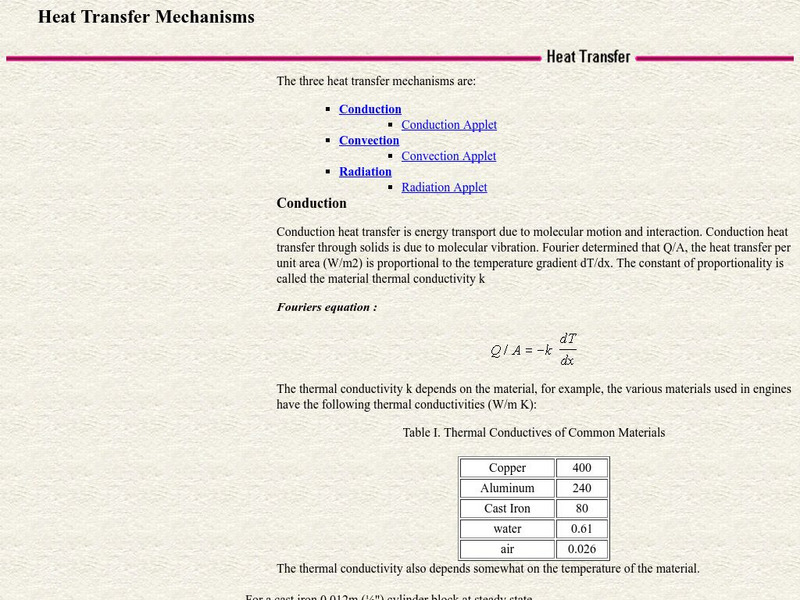
Csu: Heat Transfer Mechanisms
An excellent page from the Colorado State University with a heavy mathematical emphasis. Each form of heat transfer--conduction, convection, and radiation--is defined, compared, and contrasted. Mathematical equations governing the rates...
Colorado State University
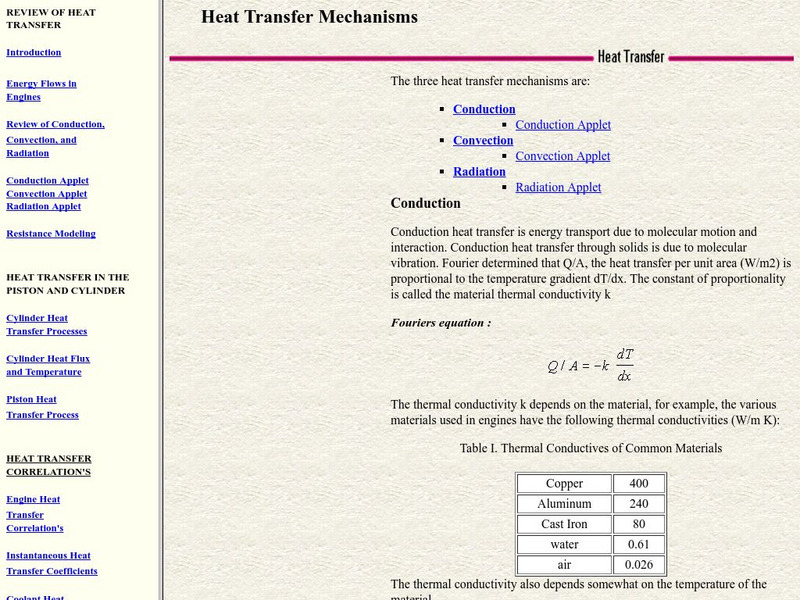
Colorado State University: Heat Transfer Mechanisms
An excellent page from the Colorado State University with a heavy mathematical emphasis. Each form of heat transfer--conduction, convection, and radiation--is defined, compared and contrasted. Mathematical equations governing the rates...
Colorado State University
Colorado State Univ.: Heat Transfer Resistance Modeling
This site from the Colorado State University discusses the tranfer of heat by conduction and convection. Discussion centers around the application of these two heat transfer mechanisms to engines. The variables that effect the resistance...
Chemicool
Chemicool: Platinum
A set of tables summarizing a great many chemical and physical properties of platinum.
BBC
Bbc: Gcse Bitesize: Covalent Bonds
A covalent bond is formed between non metal atoms, which combine together by sharing electrons. Covalent compounds have no free electrons and no ions so they don't conduct electricity.