Curated OER
Binary Compounds
Binary compounds are explained with the periodic table, atomic charges, and examples. Viewers are taught to name binary compounds and then are presented with a chart of cations and ions. Finally, they see a few photos of forms of silicon...
Curated OER
Chemistry
As the title implies, this PowerPoint defines and illustrates the basic terms involved with describing matter. Phases of matter, atomic structure, and the different categories of compounds are included. Slides are simple and uncluttered....
Curated OER
Essential Elements
A color-coded periodic table identifies organic elements, major minerals, and trace elements. Oxidation states are highlighted and types of chemical bonding are annotated. The electron energy level chart is explained. Though not all of...
Curated OER
Classification of Matter
Thirty-seven slides thoroughly shed light upon the four classes of matter: elements, compounds, mixtures, and solutions. Also covered are chemical symbols, molecules, and chemical equations. The colors and fonts chosen for the...
Curated OER
Atomic Theory
An extremely thorough presentation walks new chemists through the basics of matter. There really isn't a unifying theme, however So many topics are covered: forces, elements, atomic structure, chemical properties, compounds, quarks,...
Curated OER
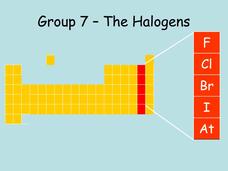
Group 7, The Halogens
The ten slides in this presentation make a consice introduction to the halogen group of the periodic table of elements. The location of the group is displayed, and then the characteristics of the different halogen group members are...
Curated OER
Atoms, Molecules and Ions
Atomic theory, experiments that contributed to our knowledge of matter, atomic structure, isotopes, and ions are covered in these 33 slides. Quality diagrams and labeled charts will help activate understanding. The presentation concludes...
Normal Community High School
Classification of Matter
Steel is an example of homogeneous mixture, also called an alloy, which is made of iron and carbon. The presentation introduces learners to elements, compounds, and mixtures. They explore their similarities and differences, and then take...
Pearson
The Chemical Context of Life
An educational presentation includes atoms, molecules, the four major elements, as well as neutrons and protons. Additionally, slides focus on atomic number, mass number, atomic weight, polar and nonpolar covalent bonding, ionic...
Curated OER
Chemistry of Carbon - Building Blocks of Life
A great review of the structure and function of carbon-based molecules important to life, especially with relevance to humans. The chemistry behind the combination of polymers and the breakdown of bonds is covered. Valuable content...
Biology Junction
Macromolecules
In chemistry, organic means something contains a carbon base. A helpful presentation starts by defining macromolecules as large organic carbon molecules. Scholars answer questions about each topic on the associated worksheet. It covers...
Curated OER
Covalent Bonding
Using chlorine as the model to demonstrate the structures and representations, this PowerPoint clearly labels and details the main atomic structures and reasons for bonding. The electrons in the outer shell are diagramed and then the...
Curated OER
Relative Mass Formula, Atomic Formula, and Empirical Formula
After giving the definitions of the different compound terms and formulas, equations are provided to teach your chemists to calculate different values. Relative formula mass, atomic mass, and empirical mass are shown and explained with...
Curated OER
Lipids: Fats and Oils
A fantastic presentation with great images should improve student understanding of lipids and their involvement in the body. The chemistry of different fats, phospholipids, and steroids are explained. Additionally, the specific...
Science Geek
Periodic Trends
If your pupils think Um is the element of confusion, this presentation on period trends can only help. It covers the patterns for atomic radii, ionization energy, and electronegativity across a period and down a group.
Curated OER
Empirical Formula
Plenty of time was invested in creating this detailed presentation. The first slide alone includes several steps that support your explanation of percent composition, a precursor to understanding how to write empirical and molecular...
Curated OER
The Mole
This compact collection of slides explains the history and use of the mole unit. Relative atomic mass, as John Dalton understood it, is explained. Avogadro's number is also introduced. The mole, or the unit for the amount of a substance,...
Curated OER
The Mathematics of Chemical Formulas
Wow! This is one of the most exhaustive presentations on finding chemical formulas that you will come across! Beginning with the basics, and leaving junior chemists with procedures and concrete examples, this PowerPoint certainly...
National Energy Education Development Project
Introduction to Hydrogen
Every region has a renewable resource that can be used to make hydrogen. But, what is hydrogen and why can it be used as an energy source? Find out with a presentation that answers these questions and then discusses where hydrogen is...
Wisc-Online
Wisc Online: Lewis Dot Structures of Covalent Compounds
Short slide show provides basic information about drawing Lewis dot structures for covalent compounds. Starts with anatomy of the atom, and then shows the relationship between atomic particles and the Periodic Table of Elements. Offers...
Sophia Learning
Sophia: Chemical Reactions: Lesson 6
This lesson will present a basic understanding of the periodic chart of elements and how to predict chemical reactions based on given information. It is 6 of 9 in the series titled "Chemical Reactions."
Sophia Learning
Sophia: Chemical Reactions: Lesson 9
This lesson will present a basic understanding of the periodic chart of elements and how to predict chemical reactions based on given information. It is 9 of 9 in the series titled "Chemical Reactions."
Sophia Learning
Sophia: Characteristics of Matter
Find out the basic characteristics and main properties of all matter.