Curated OER
Investigating the Shapes of Covalent Molecules and Measuring their Respective Bond Lengths and Bond Angles
High schoolers will draw Lewis structures of common covalent compounds using a Chem 3D computer program. They will predict the shapes of the molecules, complete a data table, and formulate rules for predicting shapes of molecules. In the...
Curated OER
Physical Science Quiz
Test your class with a physical science quiz. Learners explain chemical processes, name compounds, write formulas for chemical compounds, balance equations, and more.
Curated OER
Periodic Table
In this periodic table instructional activity, students are given 12 terms and must match the terms to their appropriate definition. Topics include types of bonds, types of elements, and types of ions.
Curated OER
Chemistry Slide Collection
A huge slide show provides a review of almost every topic there is to cover in basic chemistry! Your young scientists will be interested to see each illustration and example given. The appearance of the 120 slides varies greatly, most...
Curated OER
Bonding
In this word search worksheet, students locate 12 words related to molecular bonding. Words include molecule, ions, forces, polarity, partial, and particles.
Royal Society of Chemistry
Some A-level Reagents
Learning names and formulas can be a daunting task for young chemists, so support their study with interactive puzzles! First, users match each formula with its correct name. Then, individuals use them to complete three logic games.
Curated OER
Naming Compounds
In this naming compounds worksheet, students are given a chart to determine if the compound they are naming is ionic, covalent or polyatomic. Students practice identifying and naming ionic, covalent and polyatomic compounds. They define...
Curated OER
Naming: Putting it all Together
For this naming compounds worksheet, high schoolers are given a flow chart guiding them to name chemical compounds. They are given several different compounds to name including binary covalent and binary ionic compounds.
Santa Monica College
Lewis Structures and Molecular Shapes
Learners practice drawing Lewis dot structures, build molecules with model kits, and predict molecular shapes using VSEPR theory. The combination of written work and hands-on reinforcement benefits young scientists.
Curated OER
AP Chemistry Chapter 9
In this AP Chemistry instructional activity, students apply concepts of electron configuration to accurately answer the questions provided. Students also draw Lewis structures of the given elements. Students estimate the enthalpy of...
Virginia Department of Education
Mystery Anions
Lost an electron? You should keep an ion them. Young chemists learn qualitative analysis in the second lesson of an 11-part chemistry series. After observing reactions of simple salts, the teacher provides pupils with unknown...
Curated OER
Chapter 12 Review, Mixed Review: Solutions
Although there are only six questions on this chemistry handout, it makes a thorough review of solutions. Novices explain why a compound is not an electrolyte, identify types of compounds, and calculate moles, grams, and molalilties in...
Curated OER
Combining Atoms
In this atoms worksheet, students explore the different types of bonds, name compounds, and determine charges of molecules. This worksheet has 8 true or false, 9 fill in the blank, and 5 short answer questions.
Curated OER
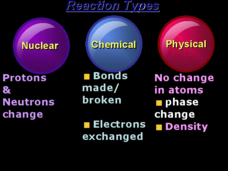
What Holds a Substance Together?
Young scholars observe how substances are held together by bonds. In this physics lesson, students demonstrate how a substance is held together by ripping newspapers and stretching marshmallows. Young scholars complete a data sheet.
Curated OER
Unit 7 Acid and Base
A series of 25 multiple choice questions is presented to chemistry learners to review properties of acids and bases. A page of notes precedes the questions and contains information about the Arrhenius and Bronsted-Lowry definitions,...
Curated OER
Size of Atoms - Trends
This compendious collection of slides leaves no questions when it comes to the concept of atomic size. Thorough and easy-to-read graphs, tables, and graphics explain atomic radii, the shielding effect, the octet rule, isoelectric...
Curated OER
Chapter 7 Vocabulary
In this online vocabulary worksheet, students match 14 definitions to the correct vocabulary term. Students may click the solution button to check answers.
Biology Junction
Chemistry
You matter—unless you multiply yourself by the speed of light squared, then you energy! Scholars learn about matter, energy, the elements and so much more using an informative presentation. Completing the included worksheet creates a...
American Chemical Society
Temperature Changes in Dissolving
Alia-Seltzer tablets cause a very obvious chemical change, but do they also cause a temperature change? Each class member explores hot/cold packs, discussing how these temperature changes occur. Groups then design and carry out their own...
Curated OER
Doing Lewis Dot Diagrams
Young scholars observe the periodic table and draw the Lewis Dot Diagram. For this investigative lesson students construct information on several elements including the Lewis Dot Formation and take a quiz on the information they...
Curated OER
How Do Atoms Stick Together?
For this chemical bonding worksheet, students answer 76 questions about compounds, Lewis dot structures, intermolecular forces between atoms, electronegativity and bonding and types of bonds.
Curated OER
Studying with Chemical Compounds
Students create a foldable to help them remember topics on chemical compounds. In this physical science lesson, students differentiate ionic and covalent compounds. Given certain compounds, they identify whether it's ionic or covalent.
Curated OER
Food Additives
In this food additives worksheet, students will read information about the use of curing salt and ion nitrates to preserve food and the health problems that can occur from using these substances. Students will then answer 4 short answer...
Curated OER
How is the Strength of an Acid Determined?
Students study acids and how they can be measured. In this acid lesson students distinguish the properties that create strong and weak electrolytes.