Hi, what do you want to do?
Curated OER
Let's Get to the Bottom of the Arctic!
Students identify the three realms of the Arctic Ocean, and describe the relationships between these realms. They describe different species associations in a benthic community.
Curated OER
Water Use and Conservation
Students discuss the different types of water found on Earth. They discover why not all water is used for drinking and calculate how much water they use. They create their own water conservation plan.
Curated OER
Earth and Matter
For this matter worksheet, students review the Earth's layers, write balanced equations, and compare the properties of different elements. This worksheet has 29 fill in the blank questions.
CK-12 Foundation
Ck 12: Percent Composition and Empirical & Molecular Formulas
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will learn to calculate the percent composition of a compound either from mass data or from the chemical formula. They will use...
CK-12 Foundation
Ck 12: Plix Series: Percent Composition
[Free Registration/Login Required] Manipulate graph data to see a representation of the percent composition of different compounds. Then answer a challenge question about the activity.
Carnegie Mellon University
Chem Collective: Composition Determination of a Mixture
In this activity, students calculate the percent composition of a mixture of two arsenic-containing minerals. Step-by-step support and feedback is provided for students who need additional help.
Frostburg State University
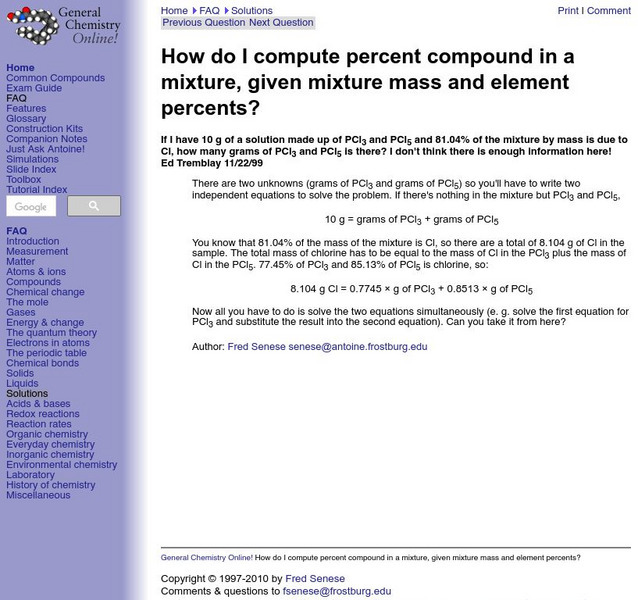
General Chemistry: Percent Compound in a Mixture
Resource contains and example problem and solution of how to compute percent compound within a mixture when given the mixture's mass and element percents.
CK-12 Foundation
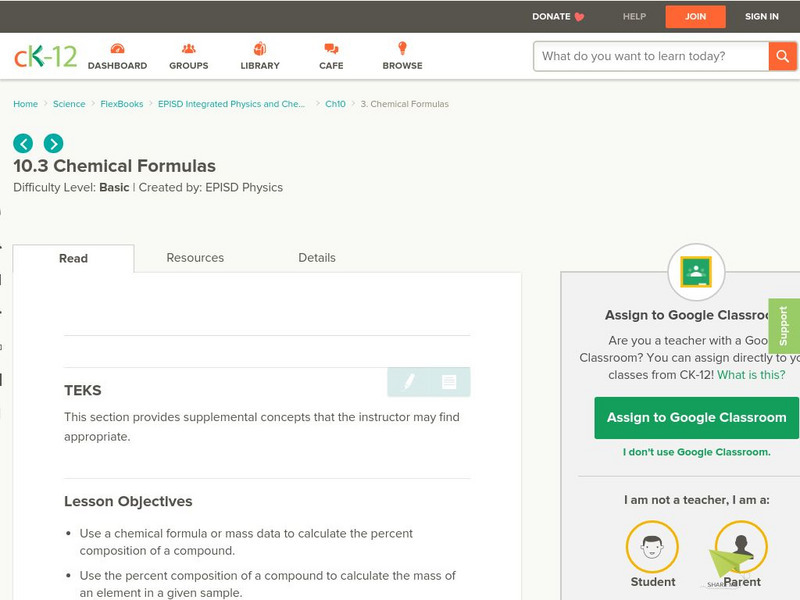
Ck 12: Chemical Formulas
[Free Registration/Login may be required to access all resource tools.] Students will use a chemical formula or mass data to calculate the percent composition of a compound, and then calculate the empirical or molecular formula for a...
Carnegie Mellon University
Chem Collective: Mineral Composition
In this randomized calculation activity, students calculate the empirical formula of a compound given its elemental analysis. Step-by-step support and feedback is provided for students who need additional help.
Science Struck
Science Struck: Composition of Air
Discover all the elements and chemical compounds that are found in the air. They are presented in a chart listing their symbols, molecular weights, and what percent of the atmosphere they each comprise. A set of facts is also provided.
Clackamas Community College
Clackamas Community College: Empirical Formulas
This site presents as explanation for calculating empirical formulas from composition data. Numerous examples are given, along with practice problems.
James Madison University
James Madison University: Igneous Rock Classification
This site explores the classification of igneous rocks. Content addresses classification based on color and texture, mineral composition, and chemical composition.
Texas Education Agency
Texas Gateway: Empirical Formula
This tutorial reviews how to calculate molecular formula by giving students video, exercises, and practice.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Stoichiometry
This online textbook chapter provides learners with advanced-level reading and practice material on stoichiometry
Towson University
Towson University: Determining Formulae
Explanation of how to calculate empirical and molecular formulas.
CK-12 Foundation
Ck 12: Chemistry Simulation: Average Atomic Mass
[Free Registration/Login Required] Students explore how percent abundances of different isotopes affect the average atomic mass for that given element. Students form connections between the average atomic mass and a person's body mass....
Other popular searches
- Percent Composition Lab
- Percent Composition by Mass
- Percent Composition Quiz
- Mass Percent Composition