Virginia Department of Education
Vapor Pressure and Colligative Properties
Hate to vacuum, but enjoy using a vacuum pump? Explore a lesson that starts with a demonstration of boiling water at various temperatures by using a vacuum pump. Then scholars design their own experiments to measure vapor pressure and...
Virginia Department of Education
Partial Pressure
At some point, everyone has been under pressure—even Dalton! Explore Dalton's law of partial pressures with young chemists as they measure the volume of air extracted from a sample compared to its original volume. Class...
Virginia Department of Education
Soap, Slime, and Creative Chromatography
Do you think chromatography paper suffers from separation anxiety? Young chemists make soap, slime, silly putty, and experiment with chromatography in this lesson. The material includes clear instructions for each experiment along with...
Curated OER
A Comparison of Land and Water Temperature
Students examine NASA satellite observations of surface temperature and investigate the seasonal changes of land and water temperature.
Creative Chemistry
Determining the Enthalpy Change of a Reaction
In this enthalpy of reactions learning exercise, students use a known amount of copper (II) sulphate solution and an excess of zinc powder to calculate the reaction's enthalpy change. Students measure the temperature change throughout...
Curated OER
California Crops
Students explore agriculture by researching the native food crops of California. Students define a list of agriculture vocabulary terms and analyze maps of California which explain which foods come from which area. Students write a...
Curated OER
Sliding and Stuttering
Ninth graders use a spring scale to drag an object such as a ceramic coffee cup along a table top or the floor. The spring scale allows them to measure the frictional force that exists between the moving cup and the surface it slides on....
Texas Education Agency
Texas Gateway: Specifically Speaking About Heat Capacity
This resource provides alternative or additional tier-one learning options to help students understand the concept of specific heat capacity.
Wikimedia
Wikipedia: Specific Heat Capacity
This Wikipedia page offers a brief description of the thermodynamics term, "Specific heat capacity."
Science Education Resource Center at Carleton College
Serc: Investigation of Heat Capacity and Specific Heat
A lab experiment where students use different temperatures of water and solids to study specific heat and heat capacity. By applying mathematical equations, students can use their data to calculate specific heat and heat capacity. Lesson...
CK-12 Foundation
Ck 12: Specific Heat
[Free Registration/Login may be required to access all resource tools.] Students explore the concept of specific heat, learn how it is calculated, and find out how different materials have different specific heat values.
OpenStax
Open Stax: Temperature Change and Heat Capacity
In this section of the textbook, find information about the transfer of heat that changes an object's temperature. Also discussed is the equation of heat transfer that relates the change in temperature, mass, and specific heat. There is...
Texas Education Agency
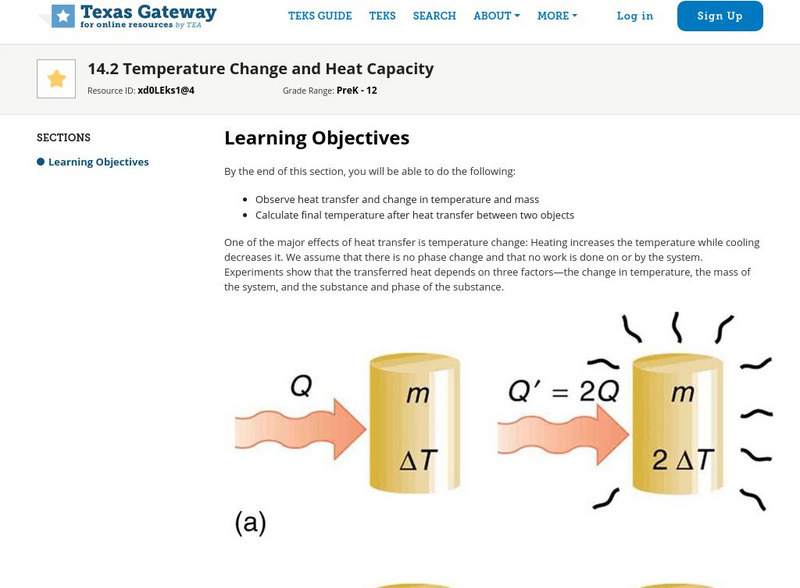
Texas Gateway: Temperature Change and Heat Capacity
By the end of this section, you will be able to observe heat transfer and change in temperature and mass and to calculate final temperature after heat transfer between two objects.
Khan Academy
Khan Academy: Biology: Specific Heat, Heat of Vaporization, and Density of Water
Why does ice float? In this article answer that question by learning about the topics of Specific heat capacity, evaporative cooling, and heat of vaporization of water.
Georgia State University
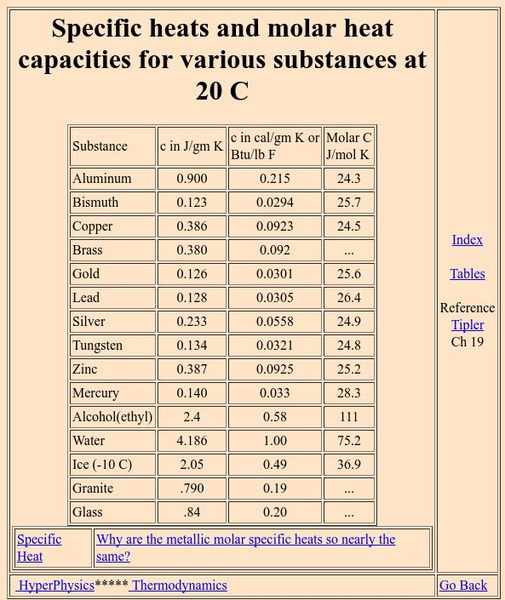
Georgia State University: Hyper Physics: Specific Heats and Molar Heat Capacities
A lengthy listing of values for specific heats and molar heat capacities for a variety of substances at 20 C. An explanation is given for why molar heat capacities for metals are nearly the same.
Simon Fraser University
Chem1 Virtual Textbook: The Heat Capacity
With an overview of topics related to chemical energetics, this site provides a foundation to a study of thermodynamics and its relation the heat capacity or specific heat.
Physics Aviary
Physics Aviary: Practice Problems: Specific Heat of a Fluid
This program asks students to determine the specific heat of a fluid based on the amount of temperature change that occurs when a hot object is placed in the fluid.
Physics Aviary
Physics Aviary: Guided Specific Heat Lab
This lab is designed to have students learn how to calculate the specific heat of a liquid based on the temperature changes that occur when hot water is added to the liquid.
Physics Aviary
Physics Aviary: Guided Specific Heat of Solid Lab
This lab is designed to have students learn how to calculate the specific heat of a solid based on the temperature changes that occur when a hot solid is added to cold water.
Chemistry Collective
Chem Collective: Measuring the Heat Capacity of an Engine Coolant.
As an analytical chemist at a company developing new engine coolants your task is to determine the heat capacity of a newly developed product and then to determine if its heat capacity is greater of less than that of ethylene glycol.
Chemistry Collective
Chem Collective: Measuring the Heat Capacity of an Engine Coolant Ii
Measure and compare the heat capacity of an unknown liquid with an unknown density.
Physics Classroom
The Physics Classroom: Thermal Physics: Measuring the Quantity of Heat
Through interactive exercises and illustrated example problems, students learn about specific heat capacity and measuring the quantity of heat.
CK-12 Foundation
Ck 12: Physical Science: Specific Heat
[Free Registration/Login may be required to access all resource tools.] Definition of specific heat and the variation in specific heat of different substances.
TeachEngineering
Teach Engineering: Heat Transfer: From Hot to Not
Young scholars learn the fundamental concepts of heat transfer and heat of reaction. This includes concepts such as physical chemistry, an equation for heat transfer, and a basic understanding of energy and heat transfer.