FuseSchool
What Is Electrolysis
Electrolysis is electrical current flow through a liquid which causes chemical changes. The liquid can be a molten ionic compound or aqueous solution. The liquid will contain free-flowing positive ions and negative ions. The positive...
Curated Video
Electrolysis of Aqueous Solutions
This video is a lecture presentation on the topic of electrolysis of aqueous solutions. It starts with a brief refresher on what electrolysis is, and then explains how electrolysis is used to split compounds using electricity. The...
FuseSchool
What Are Endothermic & Exothermic Reactions
An exothermic reaction gives off energy to the surroundings; like a fire giving off heat. An endothermic reaction takes in energy from the surroundings; like a snowman melting. Exothermic reactions transfer energy to the surroundings,...
FuseSchool
Purifying Copper
Learn the basics about Purifying copper. What methods and techniques are used in purifying copper? Find out more in this video!
Getty Images
2 pence piece being put into a solution that removes the copper, turning it silver
2 pence piece being put into a solution that removes the copper, turning it silver
Getty Images
Laser hair removal.
Closeup of woman having her facial hair removed by medical laser at beauty salon. Beautician is slowly gliding over her chin that has thin layer of gel applied onto. Low angle view. The client is wearing protective glasses.
Crash Course
Electrochemistry
Organic chemistry is difficult; those who study it have alkynes of trouble. Alkaline batteries are the focus of a video on electrochemistry, explaining how batteries work and why they are called alkaline batteries. The resource also...
Fuse School
How to Extract Aluminium Using Electrolysis
Aluminum extraction is an expensive process. A video lesson breaks down the electrolysis method of extraction. The instructor explains how to use electricity to draw aluminum out of a solution of aluminum oxide.
Fuse School
Future Extraction Methods
How do plants and bacteria help us gain access to our Earth's precious copper reserves? Scholars gain knowledge of the processes of bioleaching and phytomining in the seventh and final installment in a series discussing rocks and their...
Fuse School
Purifying Copper
Make the impure pure with a little chemistry magic! A video instructor shows how to use electrolysis and knowledge of ions to purify a sample of copper. Using background knowledge gained from the previous lessons in the 35-part series,...
Fuse School
Calculating Masses in Electrolysis
Investigate the calculation of molecular masses resulting from electrolysis of compounds. A video instructor gives a thorough explanation of mass calculations in relation to an electrolysis redox reaction. The video shows the formula,...
Fuse School
Electrolysis of Brine
Manufacture new compounds through an electrolysis process. The 29th lesson of the 35-part chemistry series explains how to decompose NaCl through electrolysis. The instructor explains the ionic implications and how to detect the...
Fuse School
How to Extract Aluminum Using Electrolysis
Newsflash: aluminum isn't born in the shape of a can! Learn the intricate process of extracting aluminum to its elemental form. Building from the previous lesson in the 35-part video series, the 28th installment explains how electrolysis...
Fuse School
Electrolysis of Molten Compounds
Charge into the next lesson in the stimulating series on electrolysis of ionic compounds! The 27th lesson of 35 focuses on the redox reactions created from electrolysis of compounds. The instructor explains the decomposition of the...
Educreations
The Electrolysis of KI
Teach the process of electrolysis of a sample compound. The video lesson explains the process of electrolysis using a common battery. Using a reduction potential table, the instructor makes a conclusion about the chemical processes at...
Educreations
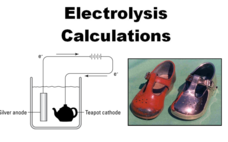
Electrolysis Calculations
Want to plate your baby shoe in copper? The video lesson explains the process of electroplating as a chemical procedure. Learners understand that electrolysis is the means to accomplish this goal.
Educreations
The Electrolysis of Water
What do you create with the electrolysis of water — and why? Answer this question and others with the in-depth video perfect for learners new to the concept. The instructor explains the significance of the positive and negative electrode...
Curated OER
Colorful Electrolysis
Watch electrolysis in action. A young student performs an electrolysis experiment as it is explained in text beneath her equipment. Show this as an example of how to perform electrolysis before having your chemistry students carry out...
Khan Academy
Khan Academy: Introduction to Electrolysis
This video compares a voltaic cell to an electrolytic cell. [6:51]
Khan Academy
Khan Academy: Quantitative Electrolysis
Calculate how much zinc deposits on the zinc electrode after 1.0 h when a current of 5.0 A is applied to the battery. [6:54]
Khan Academy
Khan Academy: Electrolysis of Molten Sodium Chloride
Understand an example of a quantitative electrolysis problem using molten sodium chloride. [12:20]
Crash Course
Crash Course Chemistry #36: Electro Chemistry
Hank discusses electrochemistry, and many of the things that go into making it possible for you to watch this episode of Crash Course Chemistry. [9:04]
Khan Academy
Khan Academy: Quantitative Electrolysis
Calculating how much zinc deposits on the zinc electrode after 1.0 h when a current of 5.0 A is applied to the battery. [7:00]
Khan Academy
Khan Academy: Electrolysis of Molten Sodium Chloride
Example quantitative electrolysis problem using molten sodium chloride. [12:23]