Educreations
Diprotic Acids
Break down an explanation of diprotic acids for your young chemists with this short instructional video. Using the example of hydrogen sulfide, Paul Groves demonstrates how to calculate the concentration of different products...
Educreations
Le Chatelier's Principle
Equilibrium reactions are able to reach a steady state of products and reactants, but what happens when this careful balance is disturbed? With the help of this instructional video, young chemists learn to apply Le Chatelier's...
Educreations
Hydrolysis
Young chemists examine the effects of salt on the pH levels of solutions with the help of this instructional video. Taking a close look at reactions between three different salts and water, students learn to predict the...
Khan Academy
Khan Academy: Keq Intuition
A probabilistic look at how molecules react to develop the intuition behind the equilibrium constant formula. [12:52]
Khan Academy
Khan Academy: Keq Derivation Intuition
A more concrete attempt at showing how the probabilities of molecules reacting is related to their concentration. [15:50]
Khan Academy
Khan Academy: Equilibrium and the Equilibrium Constant
At equilibrium, the rate of the forward reaction is equal to the rate of the backward reaction. Learn how to calculate the equilibrium constant K, the reaction quotient Q, and equilibrium concentrations. [12:01]
Khan Academy
Khan Academy: Introduction to Reaction Quotient Qc
Introduction to the reaction quotient Qc, and comparing the reaction quotient with the equilibrium constant to predict how concentrations will change. [7:36]
Khan Academy
Khan Academy: Ka and Acid Strength
Learn how to write an equilibrium expression for an acid-base reaction and how to evaluate the strength of an acid using Ka. [9:18]
Khan Academy
Khan Academy: Weak Acid Equilibrium
A quick overview of Ka and pKa. See an example of calculating the pH of a weak acid solution. [9:31]
Khan Academy
Khan Academy: 2015 Ap Chemistry Free Response 2 C
Explanation of Gibbs free energy and equilibrium constant of dehydration. Example is from the 2015 AP chemistry test. [12:23]
Khan Academy
Khan Academy: Standard Cell Potential and the Equilibrium Constant
Find the relationship between standard cell potential and equilibrium constant K. [5:18]
Khan Academy
Khan Academy: Calculate the Equilibrium Constant From Standard Cell Potential
Walk through an example problem for calculating the equilibrium constant K using the standard cell potential. [9:37]
Khan Academy
Khan Academy: Chemistry: Keq Derivation Intuition
A video lecture showing how the equilibrium constant was derived. Follow the derviation using the Haber process as an example. [15:05]
Khan Academy
Khan Academy: Chemistry: Heterogeneous Equilibrium
A video lecture explaining that heterogeneous equilibrium occurs when the chemical reaction contains molecules with different states of matter. Students will learn how set-up finding the equilibrium constant by ignoring the solvent and...
Khan Academy
Khan Academy: Chemistry: Reaction in Equilibrium
In this video module explore how equilibrium in a chemical reaction is defined as when the rate going forward is equal to the rate of the reverse reaction. Also learn how to calculate the equilibrium constant by knowing the concentration...
Sophia Learning
Sophia: Equilibrium Constant Calculation: Lesson 2
This lesson will demonstrate how to calculate the equilibrium constant of a reaction. It is 2 of 2 in the series titled "Equilibrium Constant Calculation."
Sophia Learning
Sophia: Equilibrium Constant of a Reversible Reaction: Lesson 1
This lesson will define the equilibrium constant of a reversible reaction. It is 1 of 2 in the series titled "Equilibrium Constant of a Reversible Reaction."
Bozeman Science
Bozeman Science: Reaction Quotient
In the following video Paul Andersen explains how the reaction quotient is used to determine the progress of a reversible reaction. The reaction quotient (Q) is the ratio of the concentration of products to the concentration of...
Bozeman Science
Bozeman Science: The Equilibrium Constant
In the following video Paul Andersen defines the equilibrium constant (K) and explains how it can be calculated in various reversible reactions. The equilibrium constant is a ratio of the concentration of the products to the...
Bozeman Science
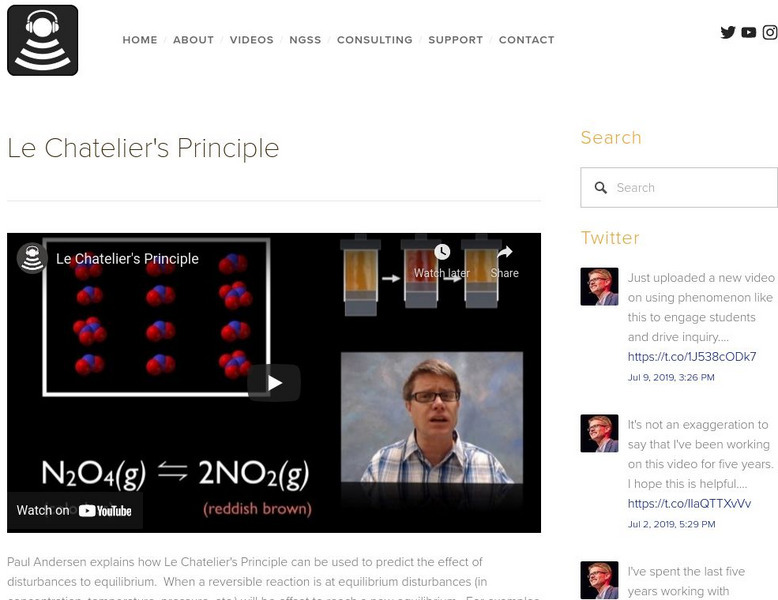
Bozeman Science: Le Chatelier's Principle
In the following video Paul Andersen explains how Le Chatelier's Principle can be used to predict the effect of disturbances to equilibrium. When a reversible reaction is at equilibrium disturbances will be offset to reach a new...
Bozeman Science
Bozeman Science: Equilibrium Disturbances
In the following video Paul Andersen explains how disturbances to a reversible reaction at equilibrium affect the equilibrium constant and the reaction quotient. For example if the concentration is changed the reaction will move to...
Bozeman Science
Bozeman Science: Free Energy and the Equilibrium Constant
In the following video Paul Andersen explains how thermodynamic and equilibrium reasoning can be related through changes in free energy and the equilibrium constant. When the delta G is negative the reaction shifts to the right or favors...
Khan Academy
Khan Academy: Chemistry: Keq Intuition
A video lecture showing how the equilibrium constant is derived. Understand how equilibrium in a reaction is reached when the forward reaction rate equals the reverse reaction rate. The video explores how knowing the concentrations of...
Khan Academy
Khan Academy: Standard Change in Free Energy and the Equilibrium Constant
The relationship between standard Gibbs free energy change and the equilibrium constant K. Calculating K when you know the standard free energy of reaction. [10:45]