Crash Course Kids
(LEGO) Block Party
Get your blocks out! It's time to start building and learning about mass! Enthusiastic chemists get in-depth information about the conservation of mass and the way it applies to chemical changes.
TED-Ed
What Triggers a Chemical Reaction?
Chemical reactions are happening all around us every second of every day, but what exactly causes these changes to occur? Using easy-to-understand analogies, this video explains how the concepts of enthalpy and entropy determine the ways...
Educreations
Entropy & Free Energy
An understanding of chemical reactions really boils down to two concepts: entropy and enthalpy. Follow along with this instructional video as it explains how these two principles are used to calculate Gibbs free-energy which...
Sophia Learning
Sophia: Energy Diagram of a Chemical Reaction
This lesson will introduce the energy diagram of a chemical reaction, and label the parts of the diagram.
Bozeman Science
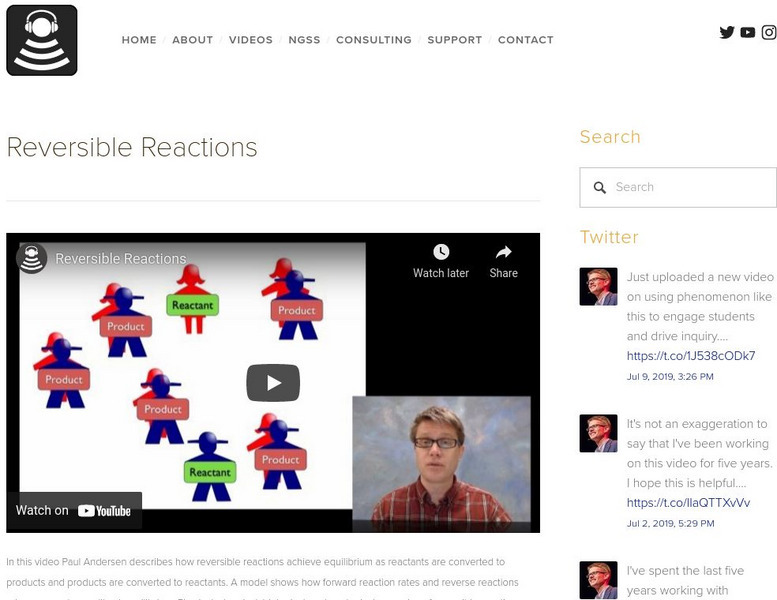
Bozeman Science: Reversible Reactions
In this video Paul Andersen describes how reversible reactions achieve equilibrium as reactants are converted to products and products are converted to reactants. A model shows how forward reaction rates and reverse reactions rates...
Bozeman Science
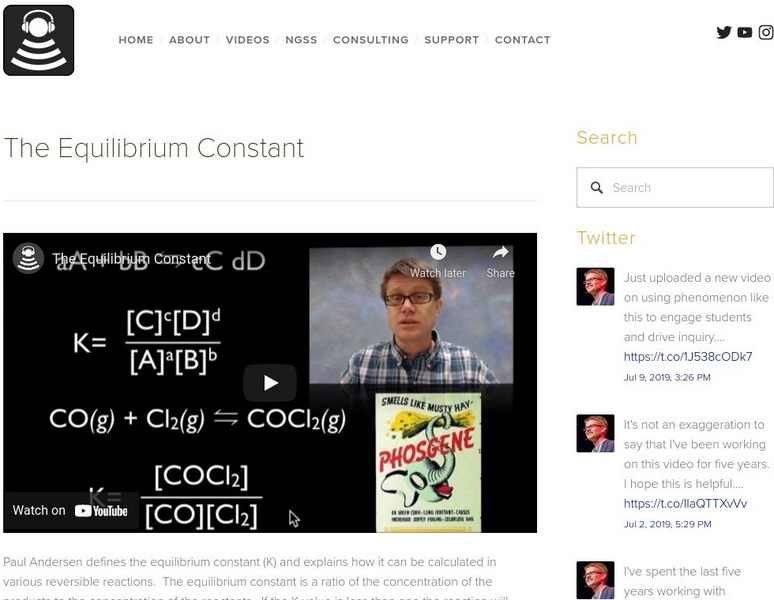
Bozeman Science: The Equilibrium Constant
In the following video Paul Andersen defines the equilibrium constant (K) and explains how it can be calculated in various reversible reactions. The equilibrium constant is a ratio of the concentration of the products to the...
Bozeman Science
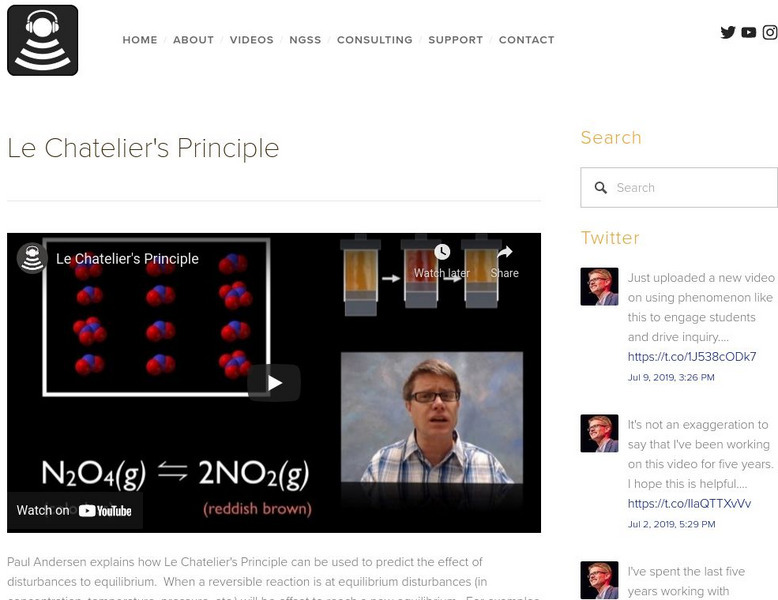
Bozeman Science: Le Chatelier's Principle
In the following video Paul Andersen explains how Le Chatelier's Principle can be used to predict the effect of disturbances to equilibrium. When a reversible reaction is at equilibrium disturbances will be offset to reach a new...
Brightstorm
Brightstorm: Science: Percent Yield
Video tutorial explains percent yield for students and provides information on how to calculate and solve chemical equations. [5:16]
Tyler DeWitt, PhD
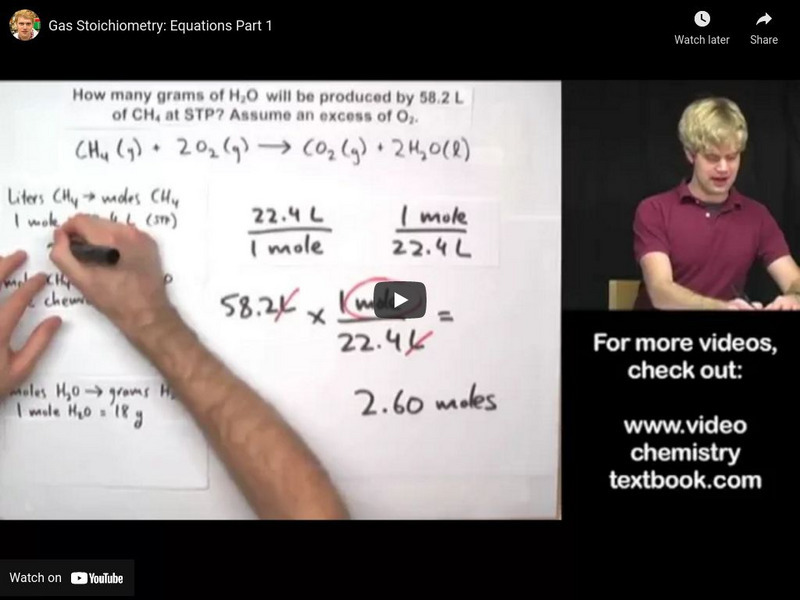
Tyler De Witt: Gas Stoichiometry: Equations Part 1
Examples and practice problems of solving equation stoichiometry questions with gases. [5:38]
Khan Academy
Khan Academy: Chemical Reactions Introduction
Reactants and products in reversible and irreversible chemical reactions. [9:12]